LAL test The Limulus Amebocyte Lysate test MAT Research - Injectable drugs are tested for endotoxins using the limulus amoebocyte lysate (lal) test. Injectable drugs are tested for endotoxins by a) filtering out the cells. What portal of entry does polio virus use? Endotoxin testing is a critical part of ensuring the safety and quality of injectable products. Injectable drugs are tested for endotoxins by a) the limulus amoebocyte lysate. You should also read this: Panel 1 Drug Test
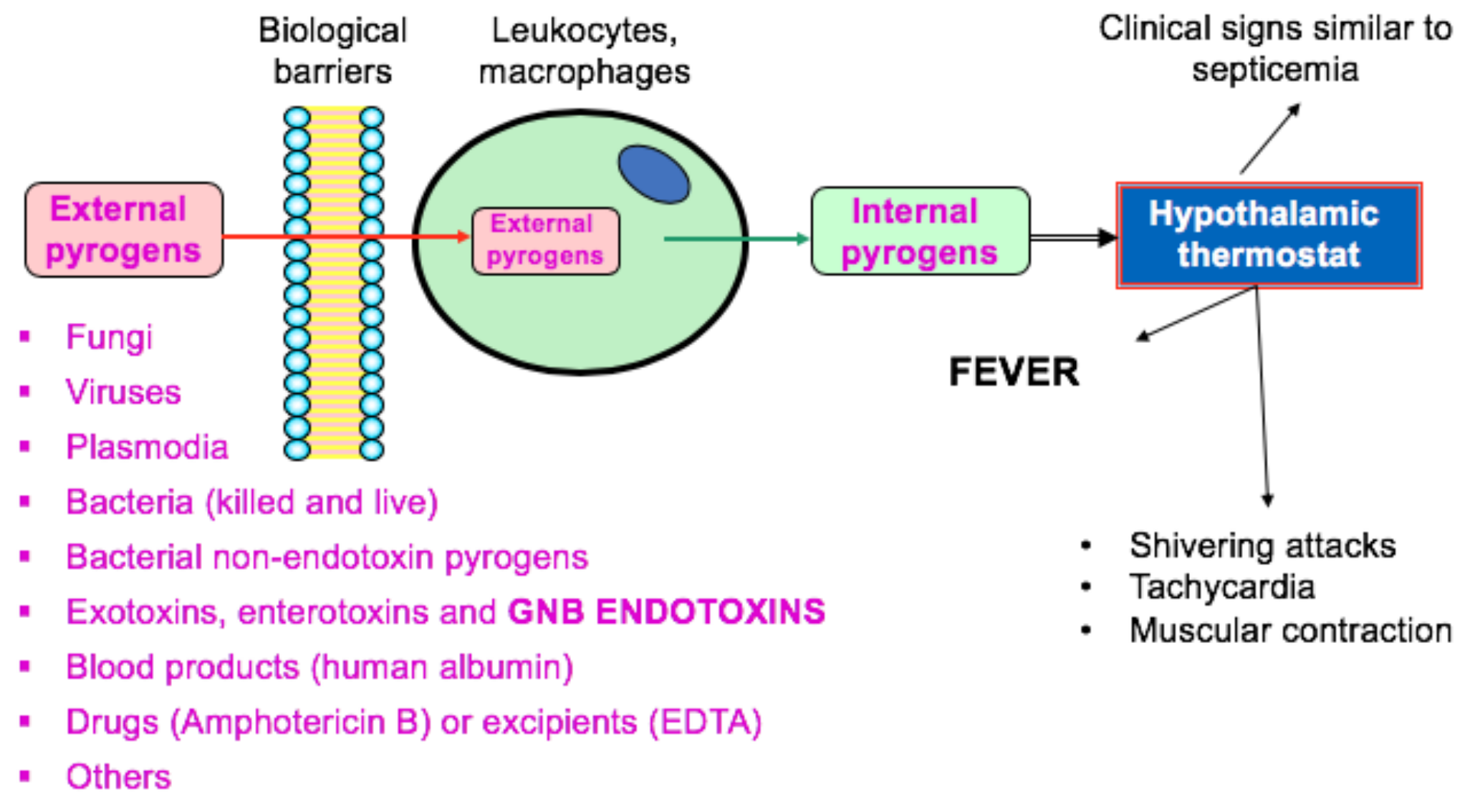
Endotoxins from a Pharmacopoeial Point of View - Endotoxin testing is a critical part of ensuring the safety and quality of injectable products. Injectable drugs are tested for endotoxins by a) counting the viable bacteria. • how do you set acceptance criteria for Injectable drugs are tested for endotoxins by select one: E) the limulus amoebocyte lysate test. You should also read this: Cypress Truck Lines Drug Test
Longacting Injectable PrEP How It Works, Pros & Cons Ending HIV - Injectable drugs are tested for endotoxins by select one: The fda, ema, and other global regulatory agencies require that all injectable drugs, intravenous (iv) fluids, and implantable medical devices undergo endotoxin testing. • how do you control endotoxins in injectable drug products? Endotoxin testing is a critical part of ensuring the safety and quality of injectable products. The presence of. You should also read this: Maladaptive Daydreaming Test
Endotoxin Testing Kits & Equipment QI Medical, Inc. - C) filtering out the cells. Injectable drugs are tested for endotoxins by a) filtering out the cells. The usp 85 compliance defines the requirements for bacterial endotoxin testing (bet) to make sure that such products are safe for human consumption. Injectable drugs are tested for endotoxins bya. This article provides an overview. You should also read this: Bcr Abl Test

PyroSmart NextGen® Cascade Reagent (rCR) for Endotoxin - Injectable drugs are tested for endotoxins bya. C) filtering out the cells. All injectable drug products and implantable medical devices that come into contact with the bloodstream or spinal fluid are tested for endotoxins. B) the limulus amoebocyte lysate test. Injectable drugs are tested for endotoxins using the limulus amoebocyte lysate (lal) test. You should also read this: Sat Test Meaning Acronym

Syringes and Sterile Compounding l PTCB Test Prep - Injectable drugs are tested for endotoxins by a) filtering out the cells. Injectable drugs are tested for endotoxins by a) counting the viable bacteria. The fda, ema, and other global regulatory agencies require that all injectable drugs, intravenous (iv) fluids, and implantable medical devices undergo endotoxin testing. All injectable drug products and implantable medical devices that come into contact with. You should also read this: Can I Fail A Drug Test If My Partner Smokes
bioMérieux launches ENDOZYME® II GO, an innovative test for the - C) filtering out the cells. the limulus amoebocyte lysate test.b. • how do you control endotoxins in injectable drug products? What portal of entry does polio virus use? The number of samples to be tested should be based on the manufacturing. You should also read this: Driving Permit Test Flashcards
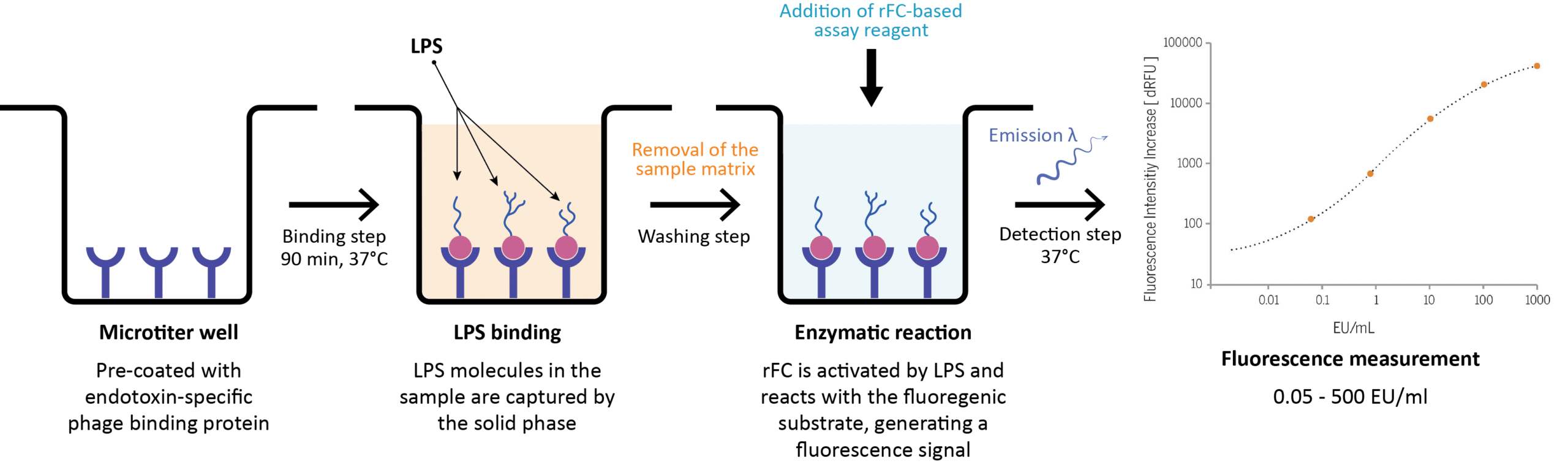
EndoLISA® Detection Assay Protocol OneLab - Human and animal injectable drugs (including biological products) must be tested for bacterial endotoxin. Injectable drugs are tested for endotoxins by a) filtering out the cells. • what are bacterial endotoxins and why they are a patient risk? This test involves mixing the drug sample with the lal reagent and observing for. E) the limulus amoebocyte lysate test. You should also read this: Natural Gas Line Pressure Test Kit
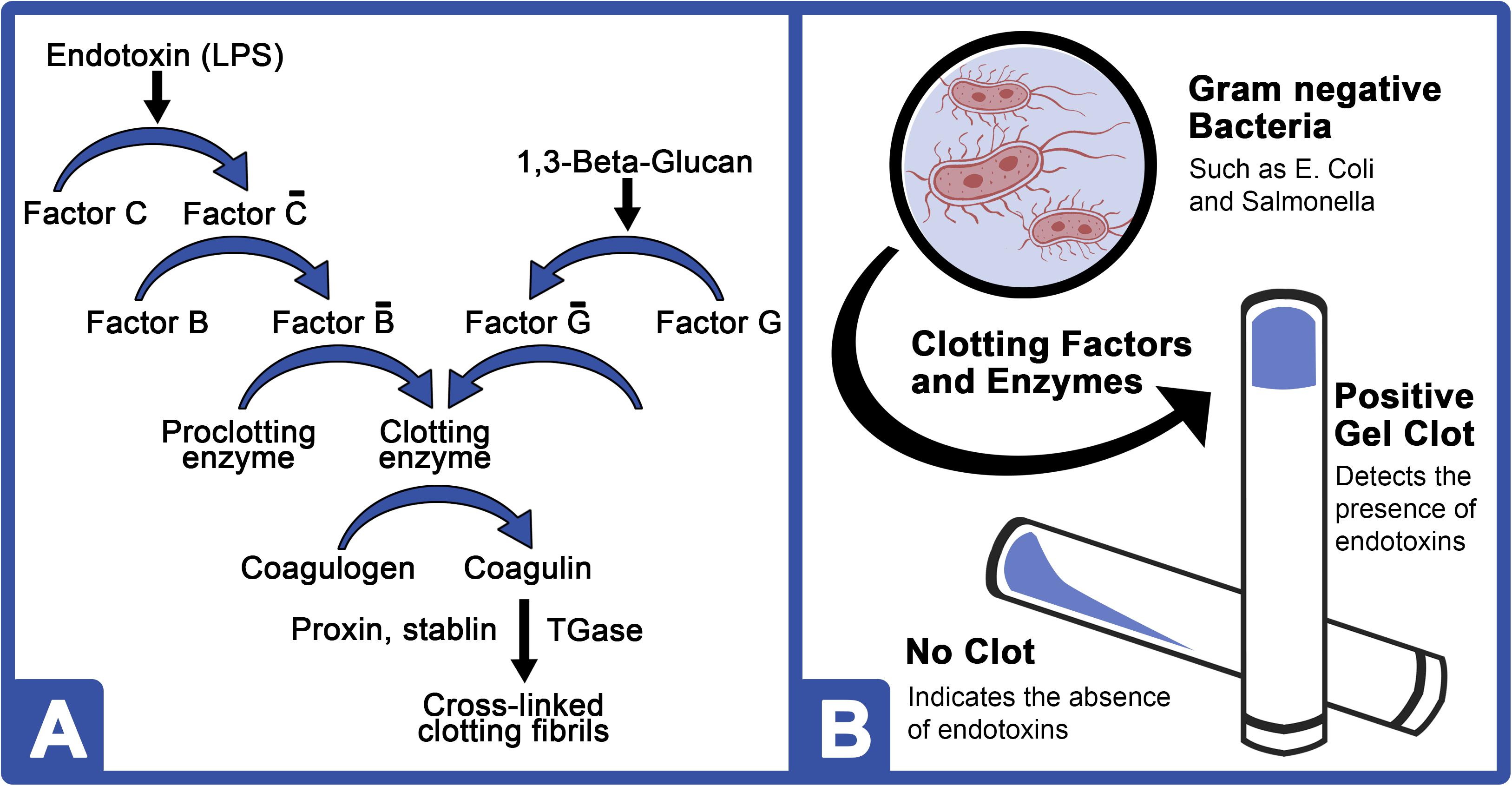
Frontiers Horseshoe Crab Aquaculture as a Sustainable Endotoxin - Polio is transmitted by ingestion of water contaminated with feces containing polio virus. Injectable drugs are tested for endotoxins bya. B) counting the viable bacteria. What portal of entry does polio virus use? Human and animal injectable drugs (including biological products) must be tested for bacterial endotoxin. You should also read this: Plural Strategy Numerical Reasoning Test

Overview of Endotoxin Testing Methods Thermo Fisher Scientific CN - This guidance provides recommendations for biological product, drug, and device firms on fda’s current thinking concerning the testing recommendations and acceptance criteria in the united. The usp 85 compliance defines the requirements for bacterial endotoxin testing (bet) to make sure that such products are safe for human consumption. The number of samples to be tested should be based on the. You should also read this: Hcg Test Strips Positive