
CLIA Compliance - Have specialized training and/or appropriate experience in molecular pathology. Our question to cap was about technical consultant requirements but thought i would add it here for reference. Minimum of 6 months experience in high complexity testing within the subspecialty of bacteriology. This subpart consists of the personnel requirements that must be met by laboratories performing moderate complexity testing, ppm procedures,. You should also read this: Walgreens Nicotine Test

PPT CLIA PowerPoint Presentation, free download ID573645 - Clinical laboratory test systems are assigned a moderate or high complexity category on the basis of seven criteria given in the clia regulations. Some specialties/subspecialties require higher educational qualifications. Bachelor’s and associate’s degrees in nursing meet the requirement for earning a degree in a biological science for, respectively, high complexity testing personnel and moderate. And (b) meet one of. High. You should also read this: Aceable Practice Test

PPT QUALITY ASSURANCE PowerPoint Presentation, free download ID1222846 - Individuals performing high complexity testing on or before april 24, 1995 with a high school diploma or equivalent with documented training may continue to perform testing only on those. List of clia requirements + responsibilities for testing personnel in the u.s. Each individual performing high complexity testing must— ( a ) possess a current license issued by the state in. You should also read this: Testa Family Dentistry

Director, Division of Laboratory Services ppt download - Documented competency assessment is required for individuals fulfilling the following personnel responsibilities outlined in subpart m of the clia regulations: Clinical laboratory test systems are assigned a moderate or high complexity category on the basis of seven criteria given in the clia regulations. I wanted to confirm that a bachelor's degree. Have specialized training and/or appropriate experience in molecular pathology.. You should also read this: Does Modafinil Show Up In A Drug Test

CLIA Compliance - And ( b ) meet one of the following requirements: Each individual performing high complexity testing must— (a) possess a current license issued by the state in which the laboratory is located, if such licensing is required; Nonwaived testing is subject to inspection, and must meet the clia quality system standards, such as those for proficiency testing, quality control and. You should also read this: A Hypothesis Is Tested With A Series Of Procedures Called

Clia Requirements Lab Director at Ronald Roche blog - Each individual performing high complexity testing must— (a) possess a current license issued by the state in which the laboratory is located, if such licensing is required; Have a minimum of 6 months of experience in high complexity testing in the applicable subspecialty. Minimum of 6 months experience in high complexity testing within the subspecialty of bacteriology. Some specialties/subspecialties require. You should also read this: Towson Prometric Testing Center

PPT QUALITY ASSURANCE PowerPoint Presentation, free download ID1222846 - Some specialties/subspecialties require higher educational qualifications. The laboratory director must be qualified to manage and direct the laboratory personnel and performance of high complexity tests and must be eligible to be an operator of a laboratory. Individuals performing high complexity testing on or before april 24, 1995 with a high school diploma or equivalent with documented training may continue to. You should also read this: The Annex Employment Testing & Wellness Center

Clia Requirements For Lab Reports at Jeff Page blog - Nonwaived testing is subject to inspection, and must meet the clia quality system standards, such as those for proficiency testing, quality control and assessment, and. One of the revised requirements in the new checklist edition is dra.10100 laboratory director qualifications, which says the laboratory director must satisfy the cap’s personnel requirements. Minimum of 6 months experience in high complexity testing. You should also read this: Lactate Poct Test
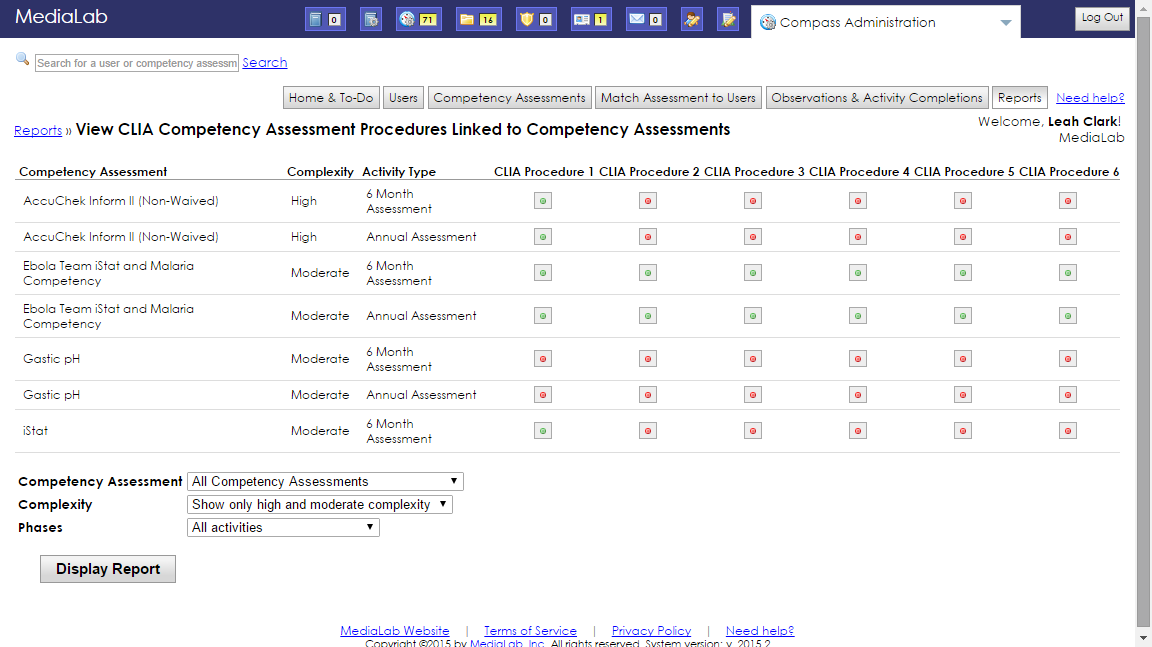
Compass for POCT & PPMP Enforce CLIA Competency Assessment Procedures - Have a minimum of 6 months of experience in high complexity testing in the applicable subspecialty. High complexity and moderate complexity laboratories. Documented competency assessment is required for individuals fulfilling the following personnel responsibilities outlined in subpart m of the clia regulations: And ( b ) meet one of the following requirements: The main difference between these 2 complexities is. You should also read this: At Home Uti Test Cvs

PPT DoD Clinical Laboratory Improvement Program (DoDCLIP) PowerPoint - I wanted to confirm that a bachelor's degree. The clia requirements are divided into 2 categories: Each individual performing high complexity testing must— ( a ) possess a current license issued by the state in which the laboratory is located, if such licensing is required; And (b) meet one of. Individuals performing high complexity testing on or before april 24,. You should also read this: North South East West Personality Test