/90191671-56a1322a3df78cf772684fbf.jpg)
Why Do Different Metals Have Different Characteristic Flame Test Colors - Flame tests are utilised in chemistry to identify the metal ions in compounds. When an electron drops from one level to a lower energy level, it emits a quantum of energy. Flame tests can be used to identify some metal ions (cations). Each metal absorbs a photon with a specific wavelength and this is what gives the flame its colour.. You should also read this: How Long Urine Can Be Stored Before Testing
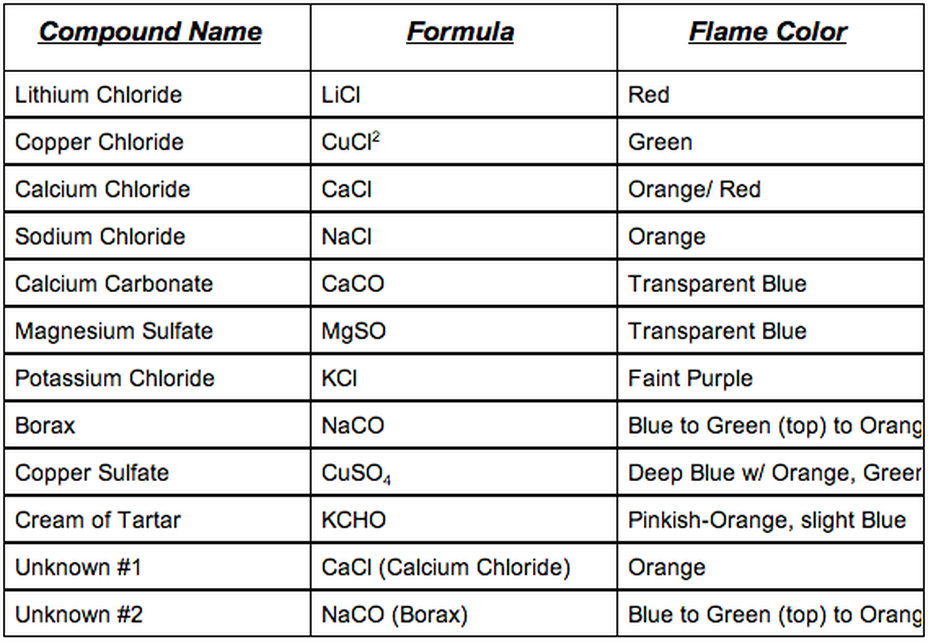
Flame Test Lab Faith's DP - The scientists can then identify their. Ever wondered why burning different metals produces a vibrant array of colors? Why do metals give off different colors in a flame test? During a flame test, chemists take an unknown metal and put it under a flame. In flame tests, salts that are dissolved in water are evaporated using a hot flame. You should also read this: What Is Non Hdl Chol In Blood Test
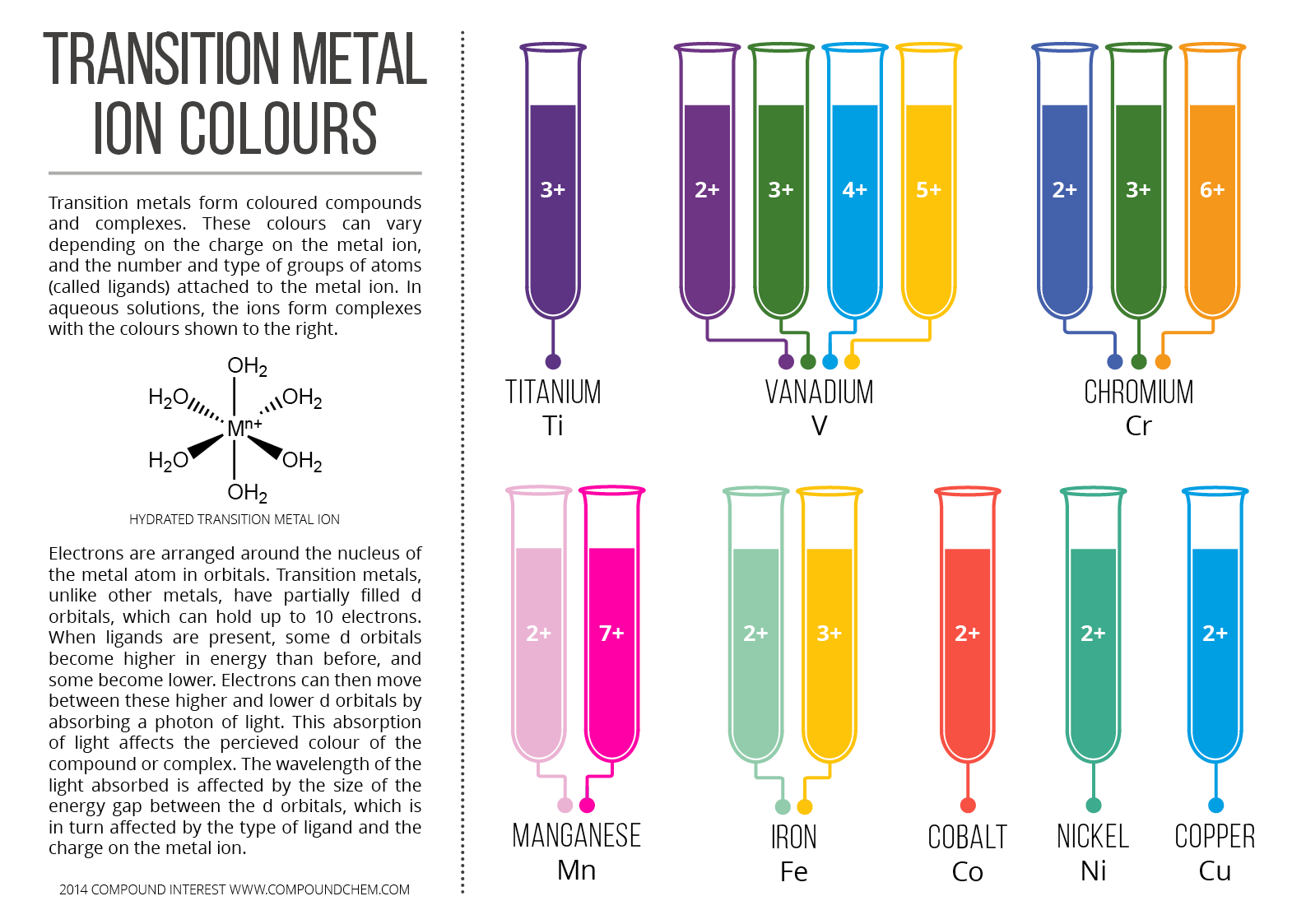
Flame Test Colors Zinc - Each metal absorbs a photon with a specific wavelength and this is what gives the flame its colour. For alkali metals, these wavelengths correspond to the colors within the visible spectrum, and therefore, are identifiable when the specific alkali metal is burned. Ever wondered why burning different metals produces a vibrant array of colors? When an electron drops from one. You should also read this: America's Test Kitchen Beef Wellington Recipe

Atomic Emission Spectroscopy & Flame Test HSC Chemistry Science Ready - Flame tests can be used to identify some metal ions (cations). The flame test is used to select metal salts that produce specific colors in fireworks. Why do metals give off different colors in a flame test? During a flame test, chemists take an unknown metal and put it under a flame. The flame test colors play a crucial role. You should also read this: Icd 10 Code For Elevated Liver Function Tests

Metal Ion Flame Test Colours Chart Compound Interest - The flame test is used to select metal salts that produce specific colors in fireworks. In the flame, the metal atoms become excited and produce their characteristic spectrum of light. Flame tests are utilised in chemistry to identify the metal ions in compounds. Is the color from the flame test a characteristic of the metal? This energy is released as. You should also read this: Written Texas Driving Test

Set Of Flame Test Colors With A Bunsen Burner Illustrations Of Various - The flame test is used to select metal salts that produce specific colors in fireworks. For alkali metals, these wavelengths correspond to the colors within the visible spectrum, and therefore, are identifiable when the specific alkali metal is burned. Flame tests are utilised in chemistry to identify the metal ions in compounds. During a flame test, chemists take an unknown. You should also read this: Positive Ober Test

Why does alkali metal imparts color to the flame test? Brainly.in - Flame tests are utilised in chemistry to identify the metal ions in compounds. Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame tests: This captivating phenomenon, known as the flame test, is rooted in the exciting world of. Is the color from the flame test a characteristic of the metal? The scientists can then identify their. You should also read this: Red Yellow Blue White Personality Test

Flame test of different metal produces different Vector Image - The flame will turn different colors based on which metal is in the substance. Metal produces a distinctive color when it lights up because the combustion. The scientists can then identify their. The flame test is used to select metal salts that produce specific colors in fireworks. By observing the characteristic colors emitted by. You should also read this: Nj Civil Service Promotional Test Results

Flame Test Color Chart - This energy is released as light, with the. Metal produces a distinctive color when it lights up because the combustion. Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame tests: The flame test colors play a crucial role in identifying and distinguishing between different metal ions in a laboratory setting. During a flame test, chemists take an. You should also read this: Tinel's Sign And Phalen's Test
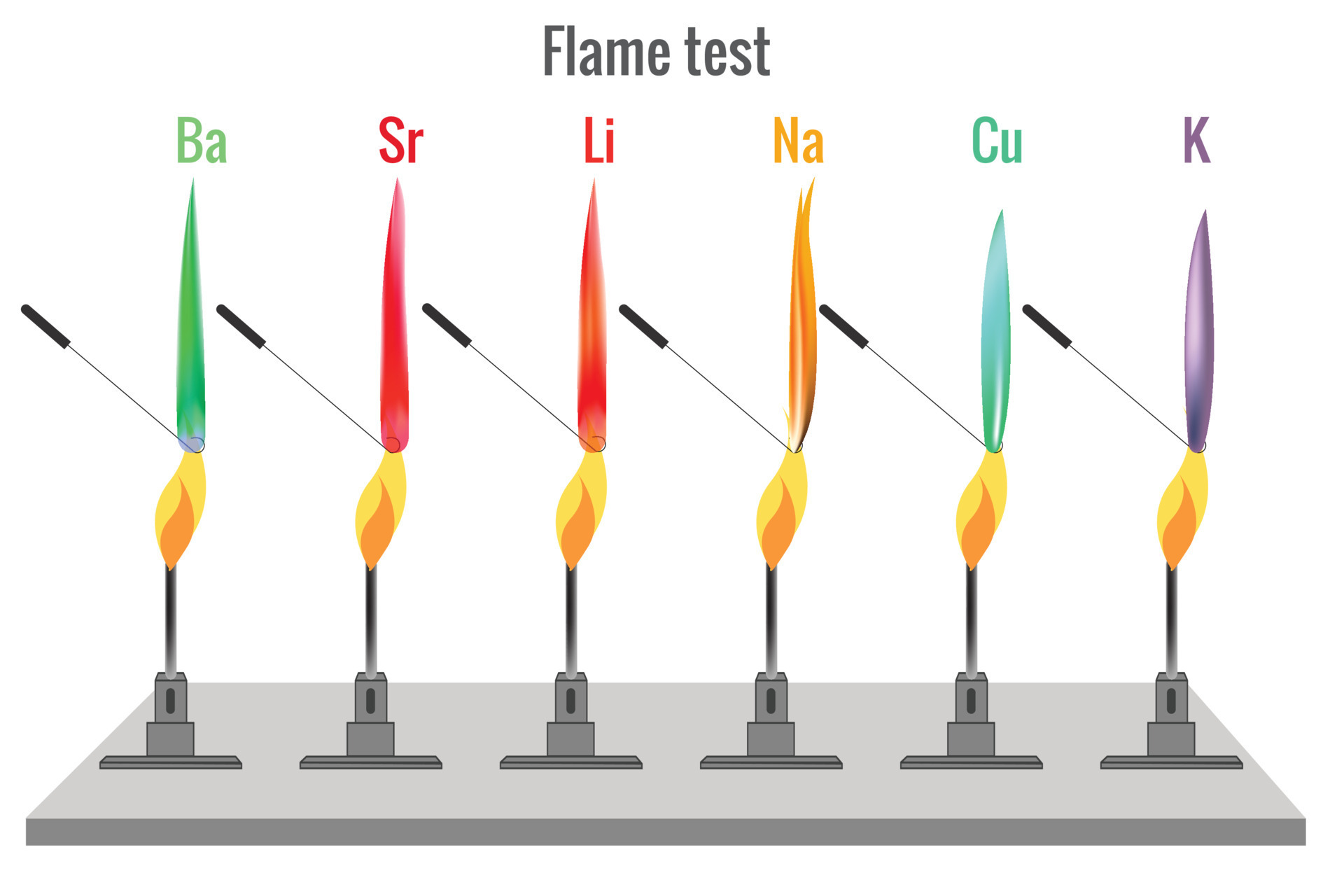
Flame test for different metal produces different color flame 23452901 - Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame tests: Is the color from the flame test a characteristic of the metal? This captivating phenomenon, known as the flame test, is rooted in the exciting world of. For alkali metals, these wavelengths correspond to the colors within the visible spectrum, and therefore, are identifiable when the specific. You should also read this: Elsevier Hesi Practice Test