
Biohubx Biomaterial Genotoxicity Assessment - The ames test, named after its creator dr. The ames test detects bacterial reverse mutations. The ames test is a commonly used method that utilizes bacteria to test whether a particular chemical can cause mutations in the dna of the test organism. A positive test indicates that the chemical is mutagenic and therefore may act as a carcinogen, because cancer. You should also read this: Reference Ranges For Blood Tests

AMES TEST YouTube - The ames test is a scientific screening experiment used to determine if a chemical is a mutagen, which induces mutations in dna. Ames test relies on the fact that certain mutated genes in bacteria convert to wild type of salmonella. It is a simple, inexpensive, and rapid assay for determining carcinogenic compounds. The ames test is a widely employed method. You should also read this: Emissions Testing Longmont Colorado
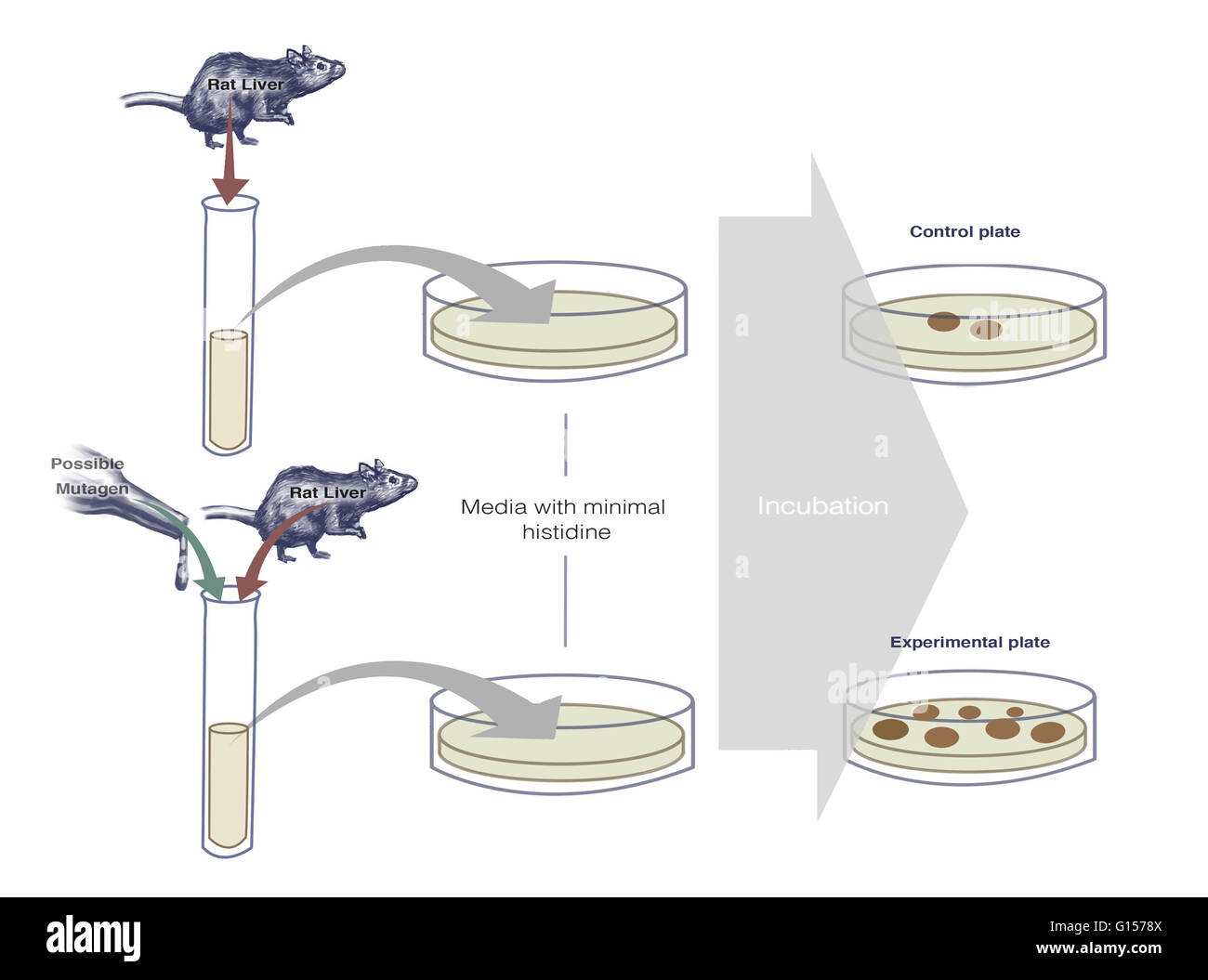
Illustration of an ames test procedure which uses bacteria to test - More formally, it is a biological assay to assess the mutagenic potential of chemical compounds. It is a simple, inexpensive, and rapid assay for determining carcinogenic compounds. The ames test is a widely accepted bacterial assay to detect the mutagenicity in pathogenic bacteria. The ames test is an in vitro genetic toxicology test designed to detect mutagenicity of chemicals by. You should also read this: Faint Line On Pregnancy Test That Disappears

Introduction, Principle, Procedure and Uses of "AMES TEST" YouTube - What controls are used in the ames test? The ames test, named after its creator dr. Ames, is a bacterial assay designed to identify substances capable of inducing mutations in dna. It gave him almost unfettered access to classified. In this protocol, although we have shown the step wise methodology to perform. You should also read this: Elisa Heartworm Test
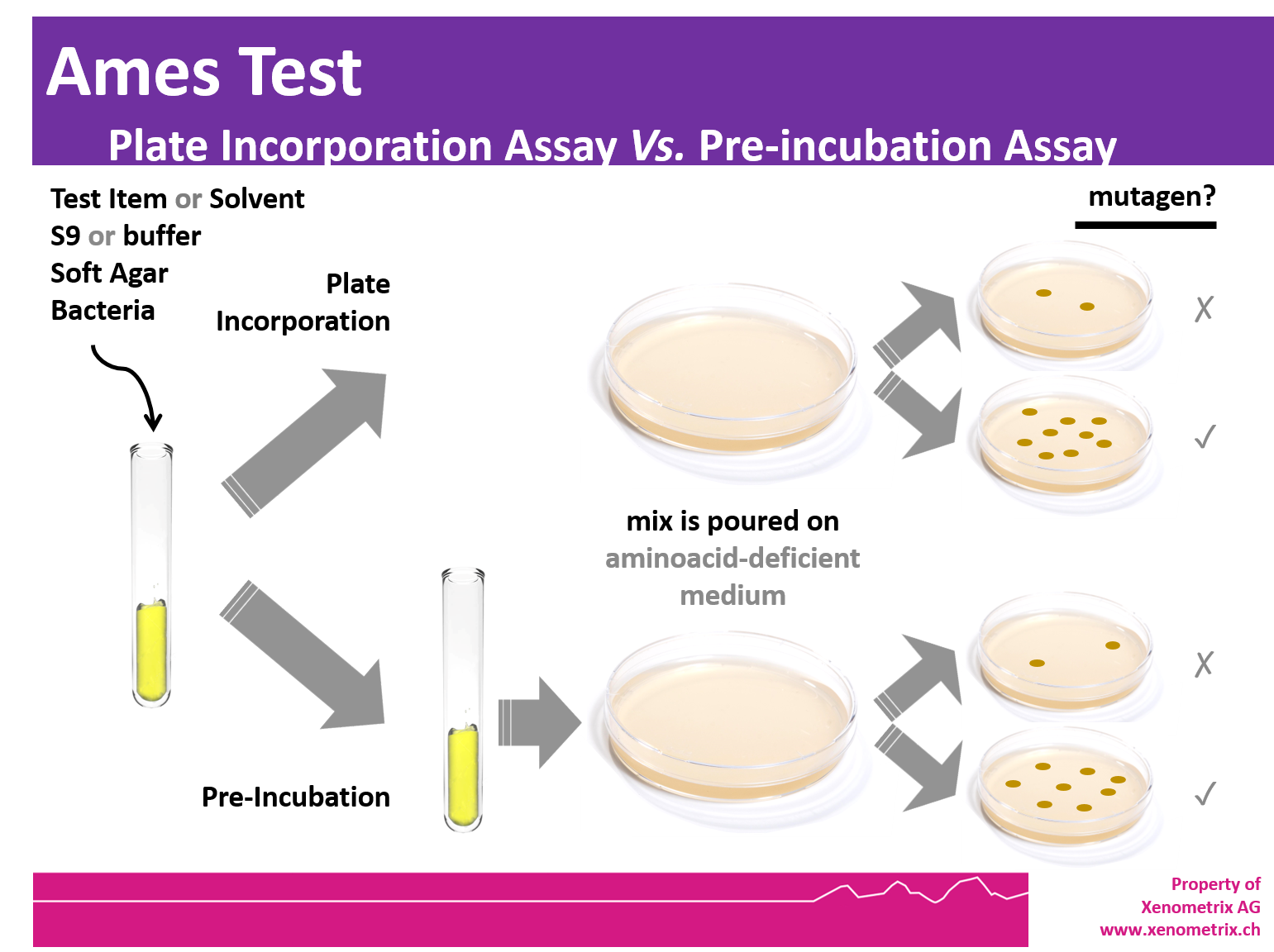
Ames Test Scientific background Xenometrix - The ames test is an in vitro genetic toxicology test designed to detect mutagenicity of chemicals by various mechanisms. The ames test is a commonly used method that utilizes bacteria to test whether a particular chemical can cause mutations in the dna of the test organism. What was the result with the round up? The bacterial strains and mutagenicity test. You should also read this: Fitnessgram Pacer Test Standards
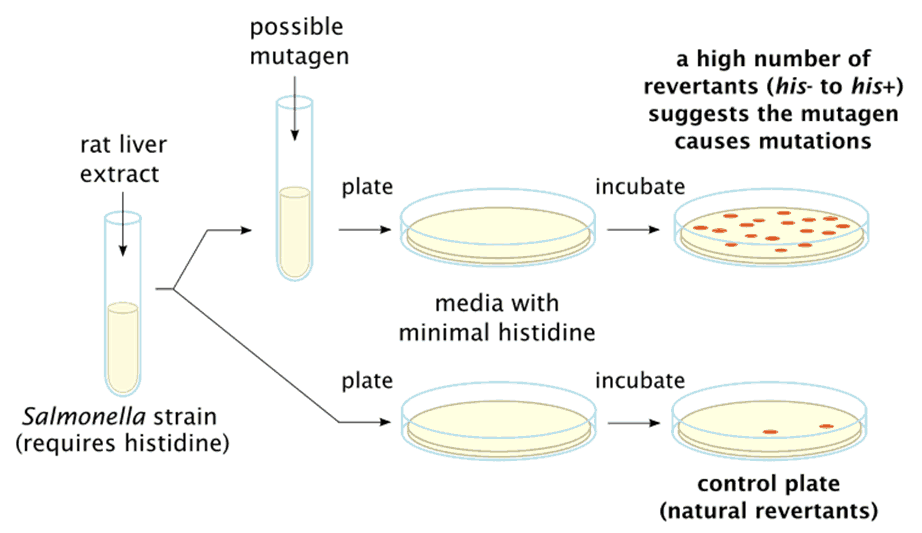
Ames Test Introduction, Principle, Procedure, Uses and Interpretation - The bacterial strains and mutagenicity test procedure developed by bruce ames, and published in 1973, greatly enhanced the ability of laboratories to test chemicals for mutagenicity. It was ames's role as head of the cia's soviet counterintelligence department that had allowed him to cause such damage. The foundation of the ames test is the idea that certain strains of salmonella.. You should also read this: America's Test Kitchen Guacamole

Ames TestMutagens & Carcinogens TestingIntroductionPrincipleBasic - The foundation of the ames test is the idea that certain strains of salmonella. The purpose of the ames test is to screen chemicals for their mutagenic properties. It gave him almost unfettered access to classified. Ames, is a bacterial assay designed to identify substances capable of inducing mutations in dna. The ames test is a widely accepted bacterial assay. You should also read this: Driving Test App Free

Ames Test Principle, Procedure, Result Interpretation, Applications - The ames test, named after its creator dr. In this protocol, although we have shown the step wise methodology to perform. It gave him almost unfettered access to classified. The bacterial strains and mutagenicity test procedure developed by bruce ames, and published in 1973, greatly enhanced the ability of laboratories to test chemicals for mutagenicity. What controls are used in. You should also read this: Washington Hca Practice Test
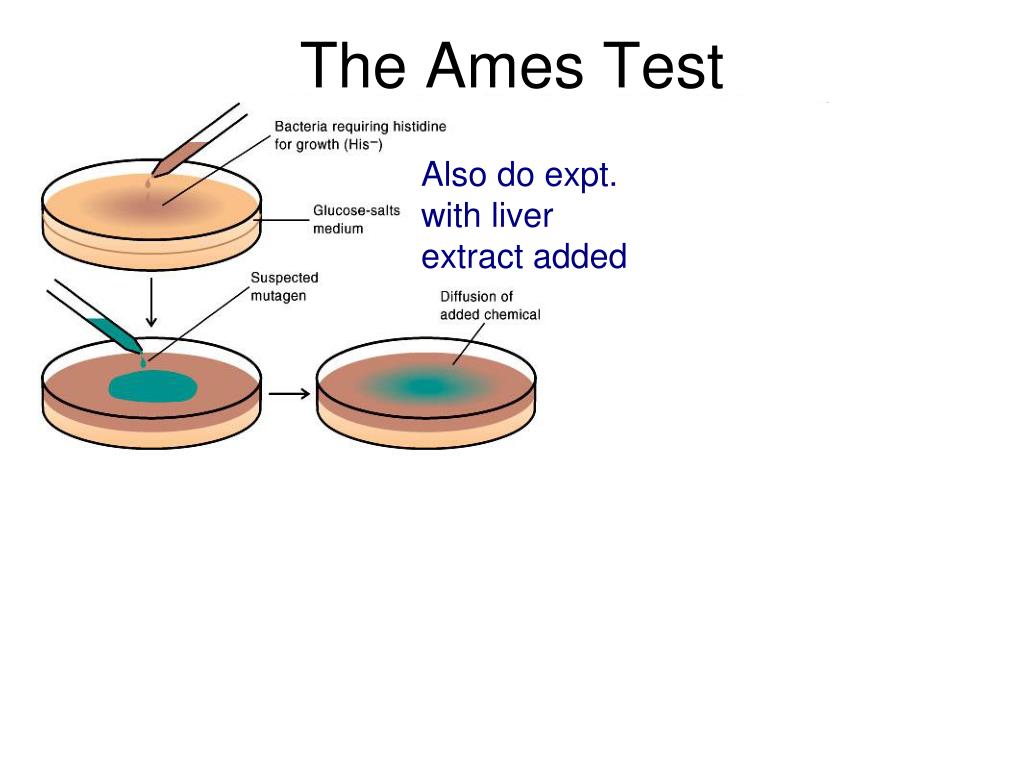
PPT Bacterial PowerPoint Presentation, free download ID - His+ revertants on the control plate are the result of. In this protocol, although we have shown the step wise methodology to perform. What was the goal of this ames test? What was the result with the round up? The ames test is a widely employed method that uses bacteria to test whether a given chemical can cause mutations in. You should also read this: Does Winn-dixie Drug Test In Florida
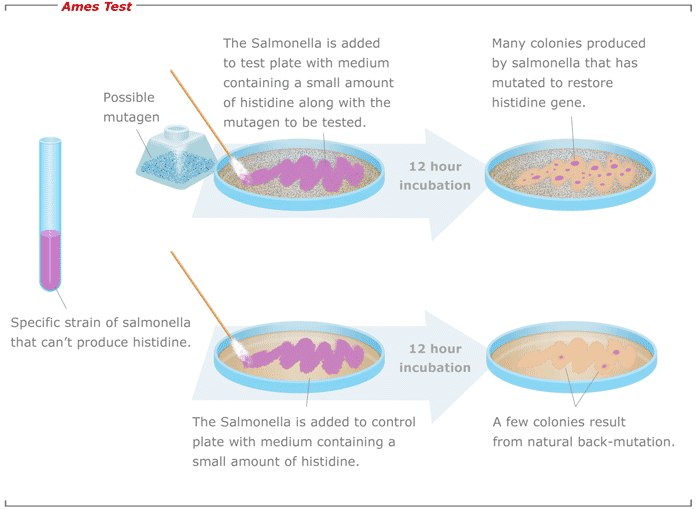
Ames testPrinciple, Procedure and Application Online Biology Notes - It was ames's role as head of the cia's soviet counterintelligence department that had allowed him to cause such damage. The ames test is a commonly used method that utilizes bacteria to test whether a particular chemical can cause mutations in the dna of the test organism. What controls are used in the ames test? A positive test indicates that. You should also read this: Consider The Test Of H0