USP Sterility Proficiency Test 40PHPT021 - All devices, with the exception of devices with pathways labeled st. Usp's revision of the standard for a sterility test has been approved by the pharmacopeial discussion group (pdg). The following procedures are applicable for determining whether a pharmacopeial article purporting to be sterile complies with the requirements set forth in the individual monograph. The test is applied to substances,. You should also read this: Xcel Testing Solutions Under Investigation

PPT USP 71 Sterility Testing of Pharmaceutical Products PowerPoint - The following procedures are applicable for determining whether a pharmacopeial article purporting to be sterile complies with the requirements set forth in the individual monograph. (please refer to the workflow for sterility test: What is usp 71 sterility testing? The test is applied to substances, preparations or articles which, according to the pharmacopoeia, are required to be sterile. Sterility testing. You should also read this: Audiometric Testing Monitors An Employee's Hearing

USPNF (71) Sterility Tests PDF - The following procedures are applicable for determining whether a pharmacopeial article purporting to be sterile complies with the requirements set forth in the individual monograph. However, a satisfactory result only indicates that no contaminating. Usp's revision of the standard for a sterility test has been approved by the pharmacopeial discussion group (pdg). The aim is to ensure the active ingredients. You should also read this: Cold Calorics Test

USP 71 Sterility Testing BA Sciences - The usp 71 sterility test is a crucial gmp microbiology testing requirement to ensure that sterile pharmaceuticals, medical devices, and radiopharmaceuticals are free from microbial. The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products,. Confirm the sterility of each sterilized batch of medium by incubating a portion of the media at the specified incubation. You should also read this: Ecpi Teas Practice Test

USP Sterility Tests Microchem Laboratory - All devices, with the exception of devices with pathways labeled st. Usp sterility testing is conducted in three primary ways for the following applications: However, a satisfactory result only indicates that no contaminating. However, a satisfactory result only indicates that no contaminating. Boston analytics provides expertise in usp method suitability for your products and.</p> You should also read this: Deq Test Station Near Me

An Inside Look at USP 71 YouTube - Boston analytics provides expertise in usp method suitability for your products and.</p> However, a satisfactory result only indicates that no contaminating. Confirm the sterility of each sterilized batch of medium by incubating a portion of the media at the specified incubation temperature for 14days.no growth of microorganisms occurs. Usp sterility testing is conducted in three primary ways for the following. You should also read this: Loep Practice Test
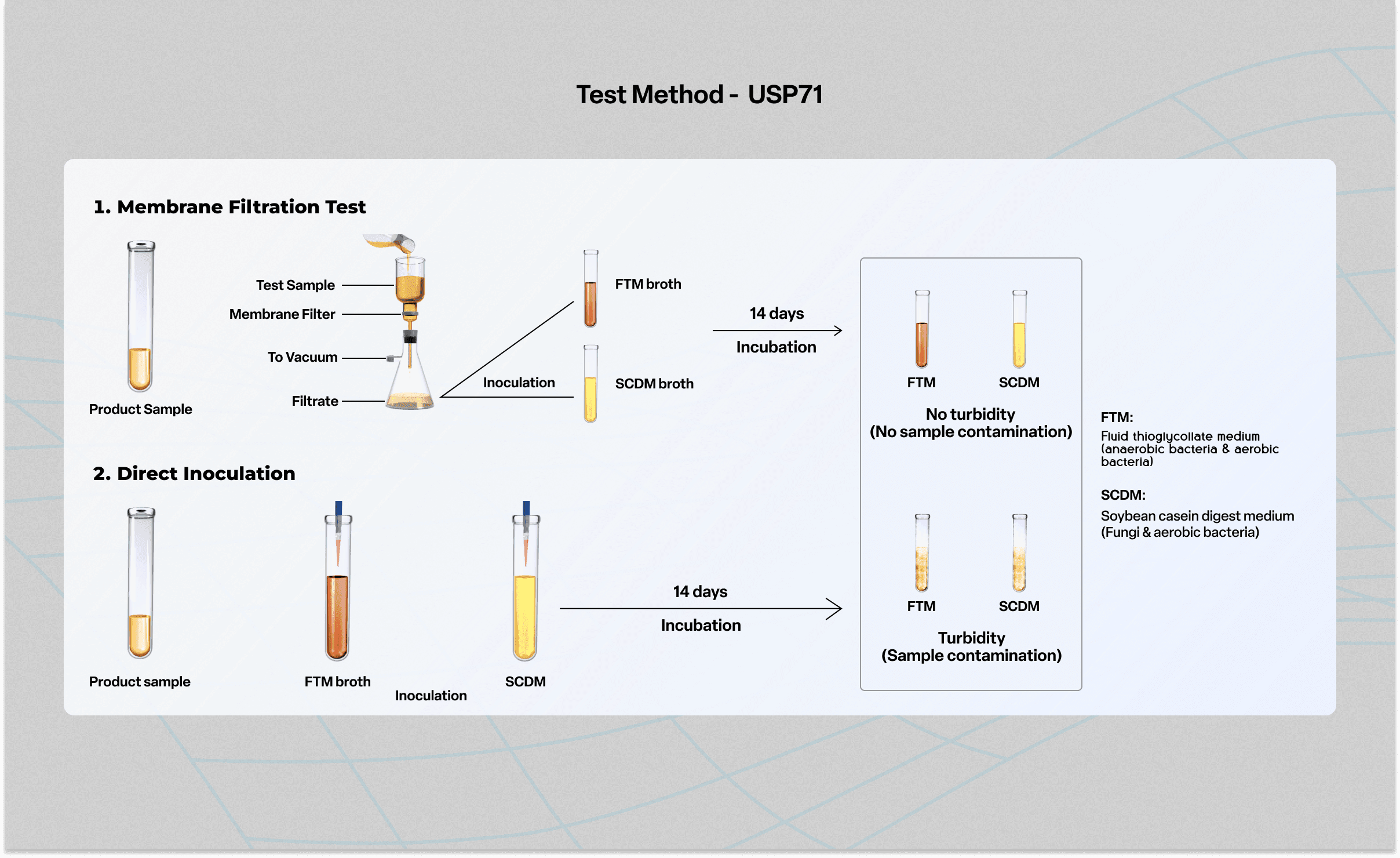
USP 71 Sterility Testing Services Microbe Investigations - (please refer to the workflow for sterility test: (71) sterility tests “portions ofthis general chapter have been harmonized with the corresponding texts of the european pharmacopeia and/or the japanese pharmacopeia. The following procedures are applicable for determining whether a pharmacopeial article purporting to be sterile complies with the requirements set forth in the individual monograph. Usp sterility testing is conducted. You should also read this: Testing Crankshaft Position Sensor

USP 71 Sterility Testing BA Sciences - Usp's revision of the standard for a sterility test has been approved by the pharmacopeial discussion group (pdg). Usp chapter sterility test states that prior to conducting a sterility test on a sterilized product, its level of bacteriostatic and fungistatic activity should be determined, that is the. The membrane filtration sterility test is the regulatory method of choice for filterable. You should also read this: Litmus Test Of A Chef Nyt

StepbyStep Guide to USP 71 Sterility Testing Methods Certified - However, a satisfactory result only indicates that no contaminating. What is usp 71 sterility testing? Boston analytics provides expertise in usp method suitability for your products and.</p> (please refer to the workflow for sterility test: Sterility testing is one of the most crucial steps in pharmaceutical product release. You should also read this: Enigma Combat Test

USP 71 Sterility Testing FOCUS Laboratories - Usp's revision of the standard for a sterility test has been approved by the pharmacopeial discussion group (pdg). Boston analytics provides expertise in usp method suitability for your products and.</p> The test is applied to substances, preparations, or articles which, according to the pharmacopeia, are required to be sterile. Usp sterility tests is a general chapter enforceable by regulatory agencies. You should also read this: Fingerprint Pregnancy Test Online Free