
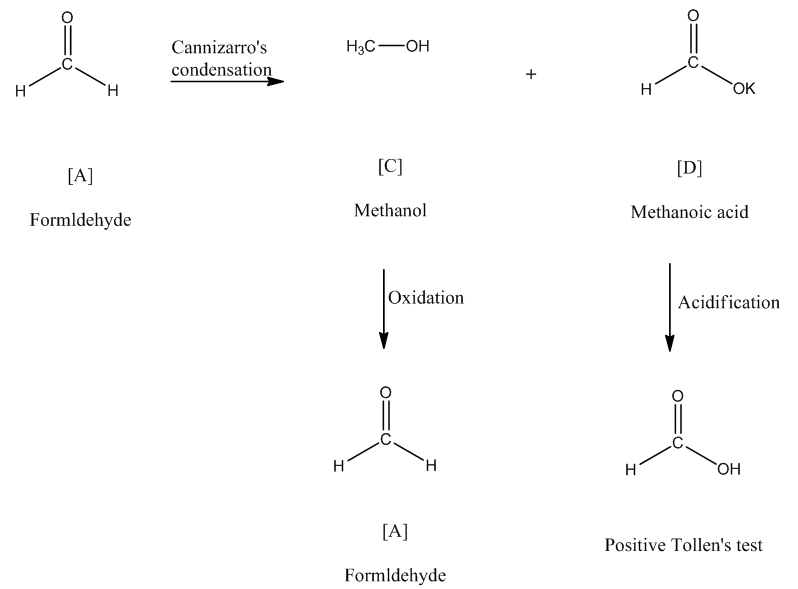
Identify total number of compounds give positive test with tollens reagen.. - Aldehydes give positive results in the tollens test and form a silver. The tollens' test indicates a positive reaction with aromatic aldehyde just like aliphatic aldehyde. A positive test with tollens' reagent is indicated by the precipitation of eleme… A positive tollens’ test is indicated by formation of dark grey precipitate or silver mirror on the bottom and sides of. You should also read this: Criterion Referenced Test Example

Which of the following compounds will give positive Tollen's test? - In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. A positive tollens test occurs when an aldehyde or alpha hydroxy ketone is present in the solution. For example benzaldehyde is oxidized to benzoic acid when tollens' reagent is added few. Tollens test, known as the. You should also read this: Bile Esculin Test Results
Tollens Reagent Test Method at Kate Terry blog - Tollens test, known as the silver mirror test, uses silver oxide and ammonia to oxidize aldehydes into carboxylic acids, producing a silver precipitate. A positive tollens test occurs when an aldehyde or alpha hydroxy ketone is present in the solution. 2agno 3 + 2naoh → ag 2 o (brown ppt) +. Under laboratory conditions, the tollens’ test is most commonly. You should also read this: Elvis Aaron Presley Jr Dna Test

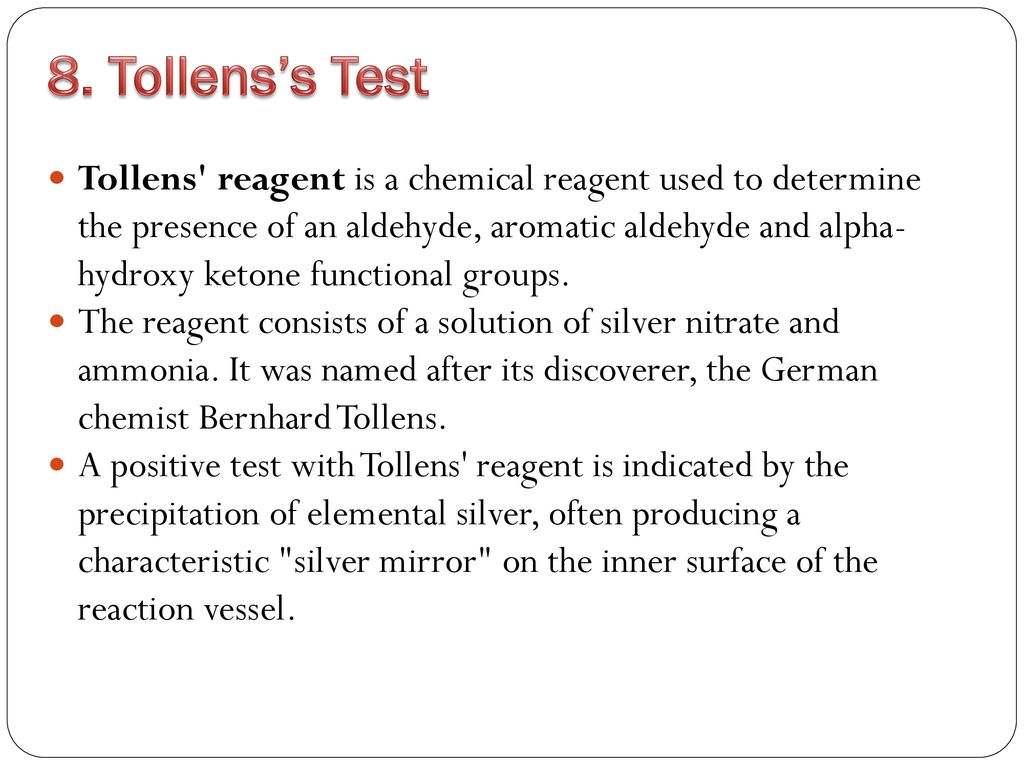
Tollens' Test or SilverMirror Test Safrole - It was named after its discoverer, the german chemist bernhard tollens. The tollens' test indicates a positive reaction with aromatic aldehyde just like aliphatic aldehyde. The reagent consists of a solution of silver nitrate, ammonium hydroxide and some sodium hydroxide (to maintain a basic ph of the reagent solution). A positive tollens’ test is indicated by formation of dark grey. You should also read this: What Evidence Supports The Cartoonist's Perspective About Testing

TEST Principles Laboratory ORGANIC CHEMISTRY LABORATORY Tollens - The reagent consists of a solution of silver nitrate, ammonium hydroxide and some sodium hydroxide (to maintain a basic ph of the reagent solution). The tollens' test indicates a positive reaction with aromatic aldehyde just like aliphatic aldehyde. It exploits the fact that aldehydes are readily oxidized (see oxidation), whereas ketones are not. Tollens test is used for distinguishing between. You should also read this: Crna Test Questions

Tollens Test - In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. It exploits the fact that aldehydes are readily oxidized (see oxidation), whereas ketones are not. It was named after its discoverer, the german chemist bernhard tollens. The tollens' test indicates a positive reaction with aromatic aldehyde. You should also read this: No Mention Of Drug Test In Offer Letter Reddit

Silver mirror test with Tollens' reagent. When a few drops of an - Tollens test, known as the silver mirror test, uses silver oxide and ammonia to oxidize aldehydes into carboxylic acids, producing a silver precipitate. The reagent consists of a solution of silver nitrate, ammonium hydroxide and some sodium hydroxide (to maintain a basic ph of the reagent solution). 2agno 3 + 2naoh → ag 2 o (brown ppt) +. A positive. You should also read this: Undertale Test Place Reborn Commands

Solved C. TOLLENS TEST Using Tollen's Reagent (a weak - What will give a positive tollens test? It was named after its discoverer, the german chemist bernhard tollens. It exploits the fact that aldehydes are readily oxidized (see oxidation), whereas ketones are not. A positive test with tollens' reagent is indicated by the precipitation of eleme… The tollens' test indicates a positive reaction with aromatic aldehyde just like aliphatic aldehyde. You should also read this: Precision Weld Testing And Training
Analysis of Carbohydrate ppt download - It exploits the fact that aldehydes are readily oxidized (see oxidation), whereas ketones are not. Tollens test, known as the silver mirror test, uses silver oxide and ammonia to oxidize aldehydes into carboxylic acids, producing a silver precipitate. A positive tollens’ test is indicated by formation of dark grey precipitate or silver mirror on the bottom and sides of the. You should also read this: 4dx Snap Test Instructions
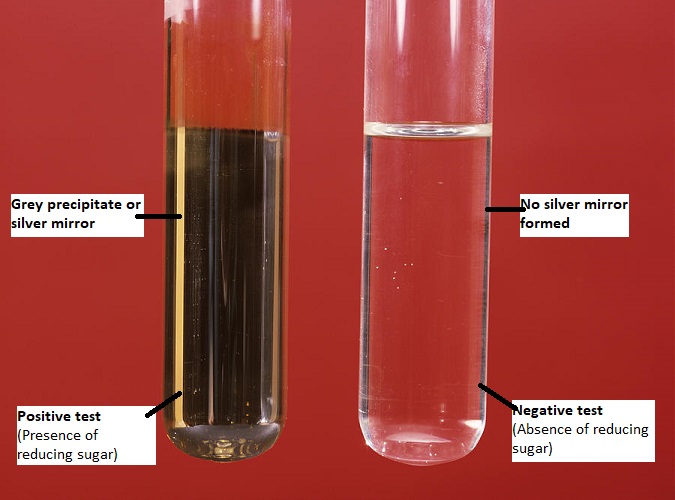
Tollen’s test or Silver mirror test Principle, Requirements, Procedure - It exploits the fact that aldehydes are readily oxidized (see oxidation), whereas ketones are not. A positive tollens’ test is indicated by formation of dark grey precipitate or silver mirror on the bottom and sides of the test tube. For example benzaldehyde is oxidized to benzoic acid when tollens' reagent is added few. 2agno 3 + 2naoh → ag 2. You should also read this: Advanced Lipid Test