
E. Testing Consumer Products For Some Cations PDF Precipitation - In this episode of keipert labs, we introduce the processes we can use in the lab to identify common cations and anions. There are 3 steps to solve this one. Qualitative analysis testing for cations and anions 4.2: Testing consumer products for some cations and anions prodact tested ation tests ot flame tests (na,k, ca2+) nh4 ts ci. Innovatech specializes. You should also read this: Sc Motorcycle Permit Test Online

Solved Testing for Cations and Anions Lab 6 D. Testing - Note that some cations do not form salts. We'll look at common tests and develop a flowchart to follow for both. There is a variety of techniques that can be used and, in the case of some organic chemistry, it can get very complicated: Cation and anions are atomic particles, so they require specialty testing procedures and equipment to identify.. You should also read this: Dat Blood Test Procedure
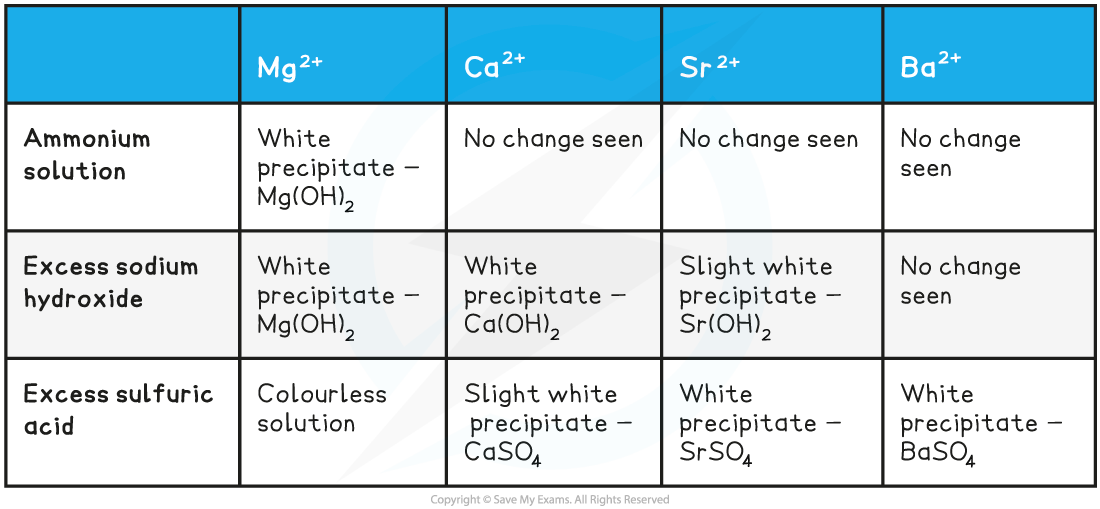
AQA A Level Chemistry复习笔记4.2.2 Identifying Anions & Cations翰林国际教育 - We'll look at common tests and develop a flowchart to follow for both. A strong persistent yellow flame indicates the presence of sodiu. Innovatech specializes in ion chromatography , a. Note that some cations do not form salts. The cations and anions in some common household consumer products will be identified by their characteristic chemical properties. You should also read this: Curo G10 Test Strips

OneClass an Testing for Cations and AnionsLab 6 ll b mbe th D - Cation and anions are atomic particles, so they require specialty testing procedures and equipment to identify. To get started, perform the flame test by exposing a clean platinum or nichrome wire dipped into the product to a bunsen burner flame and observe the color change to check for the presence. Flame tests of metal cations (experiment)” by santa monica college.. You should also read this: What To Eat Before A Pt Test
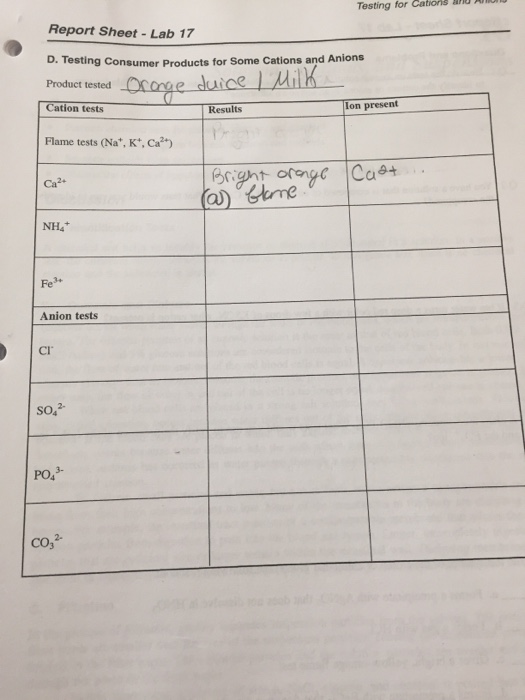
Testing for Cations Report SheetLab 17 D. Testing - Testing consumer products for some cations and anions prodact tested ation tests ot flame tests (na,k, ca2+) nh4 ts ci. When performing the analysis to determine which was the limiting reactant, a student found that the addition of bacl2â·2h2o to a test tube resulted in a. Note that some cations do not form salts. To get started, perform the flame. You should also read this: Emmersion Spanish Placement Test
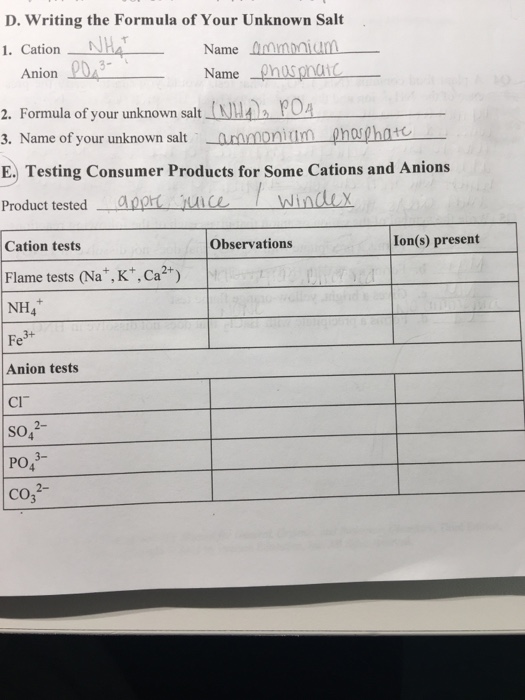
Solved D. Writing the Formula of Your Unknown Salt 1. Cation - Note that some cations do not form salts. We'll look at common tests and develop a flowchart to follow for both. When performing the analysis to determine which was the limiting reactant, a student found that the addition of bacl2â·2h2o to a test tube resulted in a. In this episode of keipert labs, we introduce the processes we can use. You should also read this: 5511 Praxis Practice Test
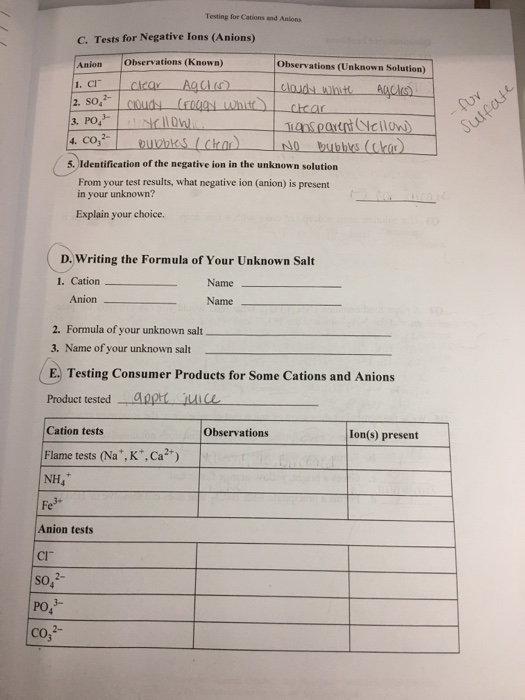
Solved Section Iestructor REPORT SHEET Testing for Cations - Looking at melting points ir spectra, nmr spectra etc. To get started, perform the flame test by exposing a clean platinum or nichrome wire dipped into the product to a bunsen burner flame and observe the color change to check for the presence. We'll look at common tests and develop a flowchart to follow for both. A strong persistent yellow. You should also read this: Exceeded Timeout Of 5000 Ms For A Test.

SOLVED Identification of the pegative lon in the unkaown solution From - Concepts to know to be prepared: We'll look at common tests and develop a flowchart to follow for both. Qualitative analysis testing for cations and anions 4.2: To get started, perform the flame test by exposing a clean platinum or nichrome wire dipped into the product to a bunsen burner flame and observe the color change to check for the. You should also read this: Nc Cdl Permit Test Answers
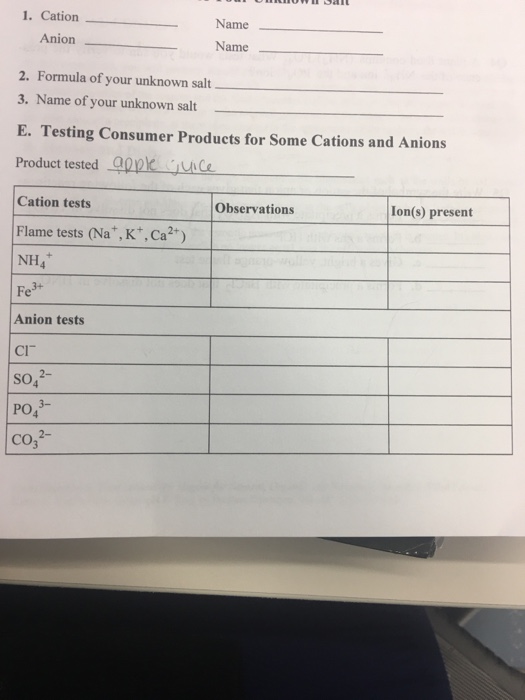
Solved E. Testing Consumer Products for Sinw Cations and - In this episode of keipert labs, we introduce the processes we can use in the lab to identify common cations and anions. The test illustrates some of the techniques and limits of qualitative analysis. When performing the analysis to determine which was the limiting reactant, a student found that the addition of bacl2â·2h2o to a test tube resulted in a.. You should also read this: Fallout 3 Test Answers
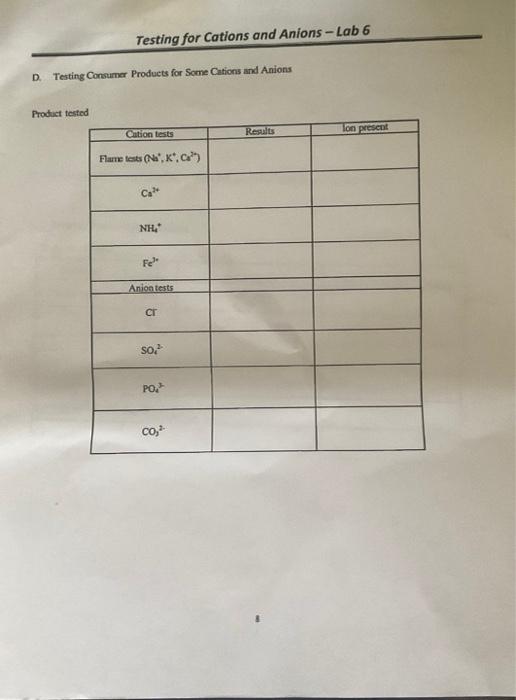
Solved Testing for Cations and Anions Lab 6 D. Testing - Concepts to know to be prepared: The test illustrates some of the techniques and limits of qualitative analysis. Innovatech specializes in ion chromatography , a. To get started, perform the flame test by exposing a clean platinum or nichrome wire dipped into the product to a bunsen burner flame and observe the color change to check for the presence. Looking. You should also read this: What Happens If You Fail The New York State Test