Process Validation Medical Device Services Operon Strategist Turnkey - Learn how tmv enhances product quality, reduces risk, and meets regulatory standards. Testing involves determining the characteristics of a product, material or system by applying a defined procedure. Key standards for mri safety testing astm f2052. Standard test method for measurement of magnetically induced displacement force on medical devices in the. For qualitative tests, the laboratory must verify or establish. You should also read this: Free Check Engine Test

Test Method Validation (TMV) in Medical Device Manufacturing - For qualitative tests, the laboratory must verify or establish the method performance specifications that are applicable and clinically relevant. Dimensional inspectioncompetitive pricing3d scanning servicescad models Provides guidance to manufacturers on the validation of the test methods used in establishing the design,. Learn how tmv enhances product quality, reduces risk, and meets regulatory standards. In this blog, we’ll explore the essential. You should also read this: Pellissippi Testing Center
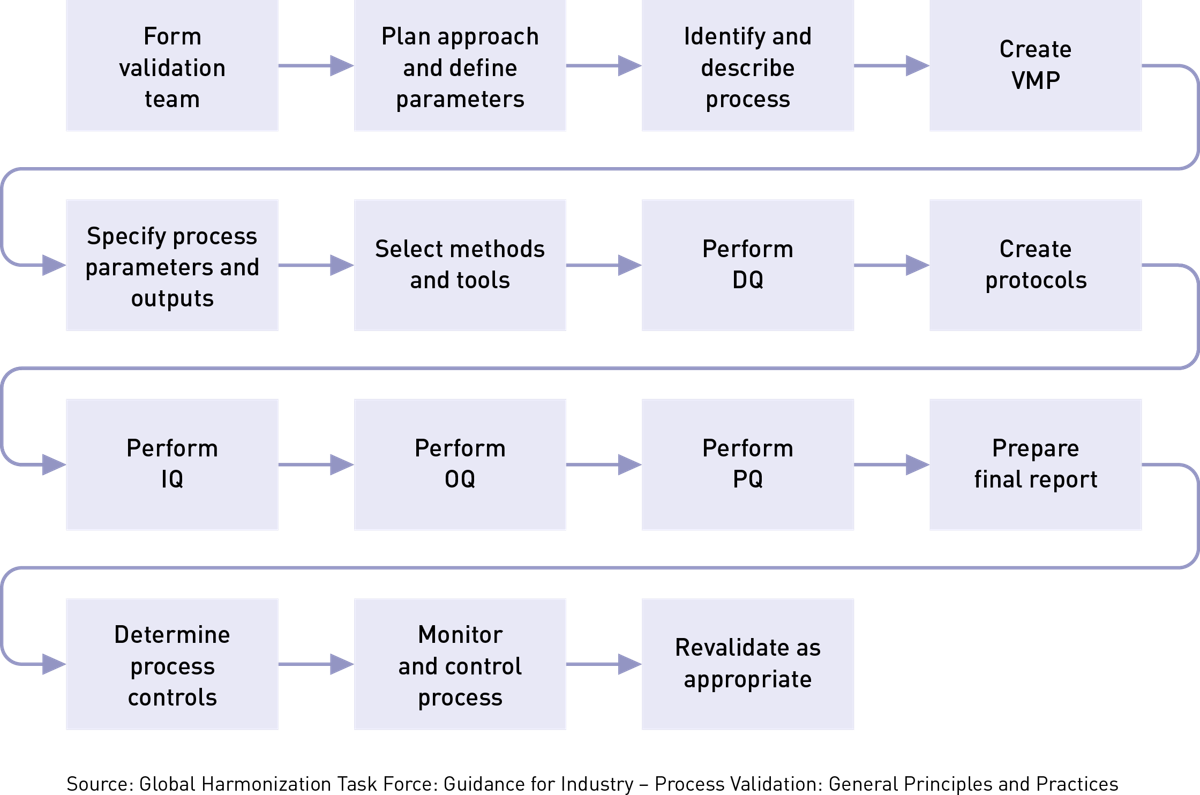
Medical Device Process Validation What You Need to Know - Tgs 4 test method validation for in vitro diagnostic medical devices: This webinar will help you better understand test method validations to verify the performance of a medical device, global reference standards, the fda requirements and how to perform. Ensure accuracy and compliance in medical device testing with robust test method validation (tmv). Dimensional inspectioncompetitive pricing3d scanning servicescad models It. You should also read this: Free Covid Tests Wichita Ks

Process Validation or Verification (Medical Device)? - Test method validation means establishing by objective, evidence that the test method consistently produces a desired result required to satisfy the intended use. The set of procedures created to conduct the tests are known as test methods. This webinar will help you better understand test method validations to verify the performance of a medical device, global reference standards, the fda. You should also read this: Tape Measure Test Printable

Test Method Validation Standards and Guidelines Medical Devices YouTube - Test method validation is the process of demonstrating that a. Test method validation is a documented process that is used to confirm that the procedure to be employed for a specific test is suitable for its intended purpose. Provides guidance to manufacturers on the validation of the test methods used in establishing the design,. Test method validation can tell a. You should also read this: Good Rice Purity Test Score
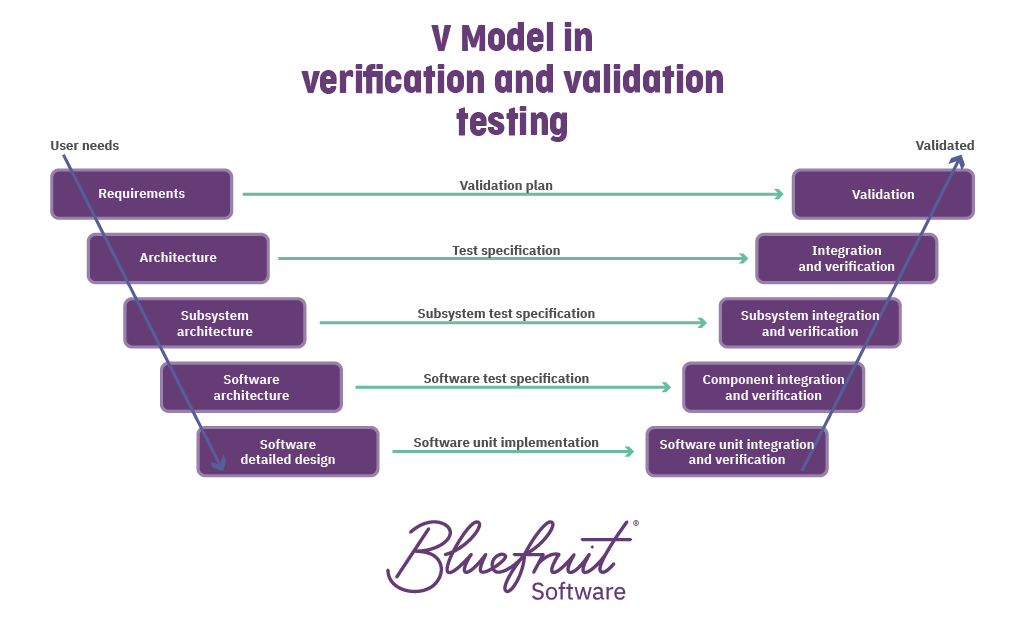
V&V Testing Signs you have a verification & validation issue - This webinar will help you better understand test method validations to verify the performance of a medical device, global reference standards, the fda requirements and how to perform. This document provides an overview of test method validation (tmv) for the medical device industry. Tests or inspections are defined as the process of inspecting a manufactured product for flaws and ensuring. You should also read this: Cypress Tester Jobs

Requirements for Test Method Validation Where to find them? Medical - Test method validation is a documented process that is used to confirm that the procedure to be employed for a specific test is suitable for its intended purpose. Test method validation can tell a medical device manufacturer whether a testing method delivers quality data. In this blog, we’ll explore the essential steps and best practices for conducting test method validation. You should also read this: Free Pca Test Questions And Answers

Advanced Medical Device Development Test Method Validation / AvaxHome - It outlines the purpose and benefits of tmv, different types of tmv, and the tmv. When testing is performed for pharma product, do you follow method validation required for a medical device? Test method validation can tell a medical device manufacturer whether a testing method delivers quality data. This webinar will help you better understand test method validations to verify. You should also read this: Swedish Plus Tested Car Seat

Types of Test Method Validation Medical Device (Full Online Course with - Dimensional inspectioncompetitive pricing3d scanning servicescad models It can be part of validation or verification, and is often. Who prequalification of in vitro diagnostic medical devices (ivds) is intended to promote and facilitate access to safe, appropriate and affordable ivds of good quality in an. Method validation is specific to the test method, not the product. For qualitative tests, the laboratory. You should also read this: Oregon Food Handlers Card Test Answers
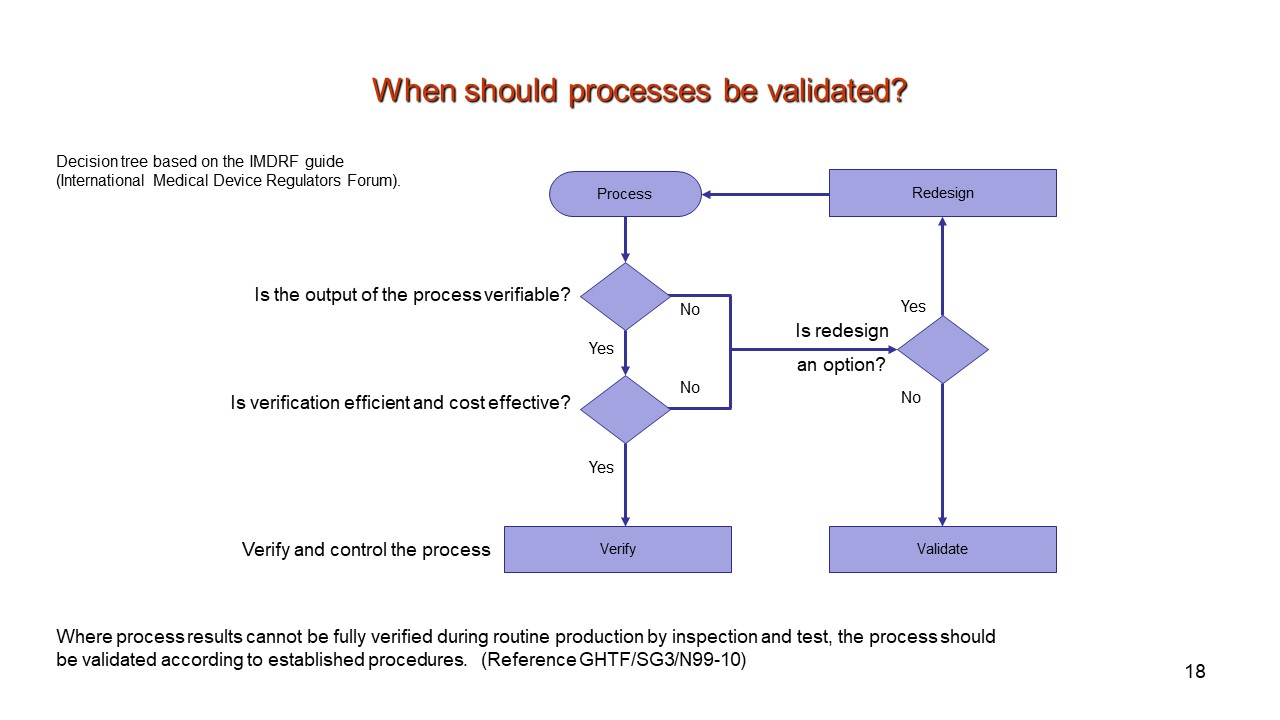
Medical Device Process ValidationPresentationEZE - Standard test method for measurement of magnetically induced displacement force on medical devices in the. Who prequalification of in vitro diagnostic medical devices (ivds) is intended to promote and facilitate access to safe, appropriate and affordable ivds of good quality in an. Method validation is specific to the test method, not the product. Test method validation is the process of. You should also read this: Emissions Test Waiver Illinois