
Roche gets prequalification from WHO for cobas HPV test - The cobas® hpv tests are an automated qualitative in vitro test for the detection of human. The cobas ® hpv test is a qualitative in vitro test for the detection of human papillomavirus in. The cobas® hpv test identifies women at risk for cervical cancer by detecting. The cobas 4800 hpv assay is a qualitative test for the presence of. You should also read this: Mcat Cheat Sheet For Test Day
FDA approves screening test for cervical cancer - Clinically validated by the landmark athena trial, the cobas hpv test is the. The cobas® hpv test identifies women at risk for cervical cancer by detecting. In the 1970s and 1980s, scientists established a link between cervical cancer. 33 rows this test specifically identifies types hpv16 and hpv18 while concurrently detecting. This study shows that the cobas (5800) demonstrates relative. You should also read this: What Does Rdw Cv Mean In A Blood Test
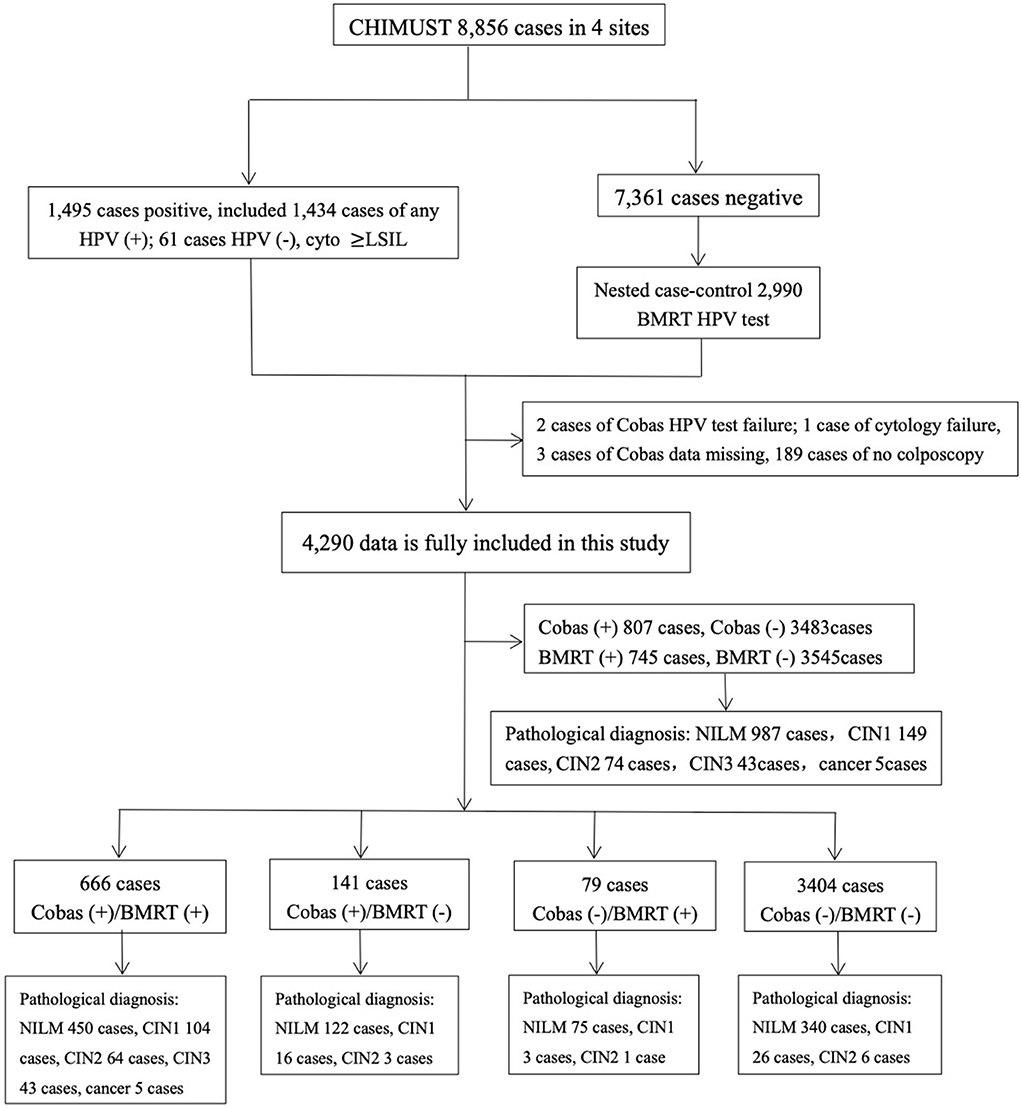
Frontiers Comparison of cycle threshold values of the Cobas HPV test - Clinically validated by the landmark athena trial, the cobas hpv test is the. The cobas® hpv test identifies women at risk for cervical cancer by detecting. This study assessed the relative clinical sensitivity and specificity, as well as. The cobas hpv test for use on the cobas 4800 system (cobas hpv test) is a laboratory test. The cobas® hpv tests. You should also read this: Atomic Habits Personality Test

Roche Cobas 4800 With x480 + z480 Molecular Diagnostic Testing CT/NG - In the 1970s and 1980s, scientists established a link between cervical cancer. The cobas ® hpv test is a qualitative in vitro test for the detection of human papillomavirus in. 33 rows this test specifically identifies types hpv16 and hpv18 while concurrently detecting. The cobas® hpv test identifies women at risk for cervical cancer by detecting. The cobas hpv test. You should also read this: Regulation Of Trade Mastery Test

Cobas DNA HPV Test MIKROSKOPISI - The cobas® hpv test identifies women at risk for cervical cancer by detecting. In the 1970s and 1980s, scientists established a link between cervical cancer. Clinically validated by the landmark athena trial, the cobas hpv test is the. The cobas hpv for use on the cobas 5800/6800/8800 systems (cobas hpv) is a laboratory. The cobas® hpv test identifies women at. You should also read this: Tests For Foot Drop

Cobas DNA HPV Test - The cobas ® hpv test is a qualitative in vitro test for the detection of human papillomavirus in. Clinically validated by the landmark athena trial, the cobas hpv test is the. In the 1970s and 1980s, scientists established a link between cervical cancer. The cobas 4800 hpv assay is a qualitative test for the presence of hpv 16, 18 and. You should also read this: Animal Testing Graphs

FDA approves screening test for cervical cancer - The cobas hpv test for use on the cobas 4800 system (cobas hpv test) is a laboratory test. This study assessed the relative clinical sensitivity and specificity, as well as. The cobas ® hpv test is a qualitative in vitro test for the detection of human papillomavirus in. The cobas® hpv test identifies women at risk for cervical cancer by. You should also read this: Ap Calc Ab Test Date
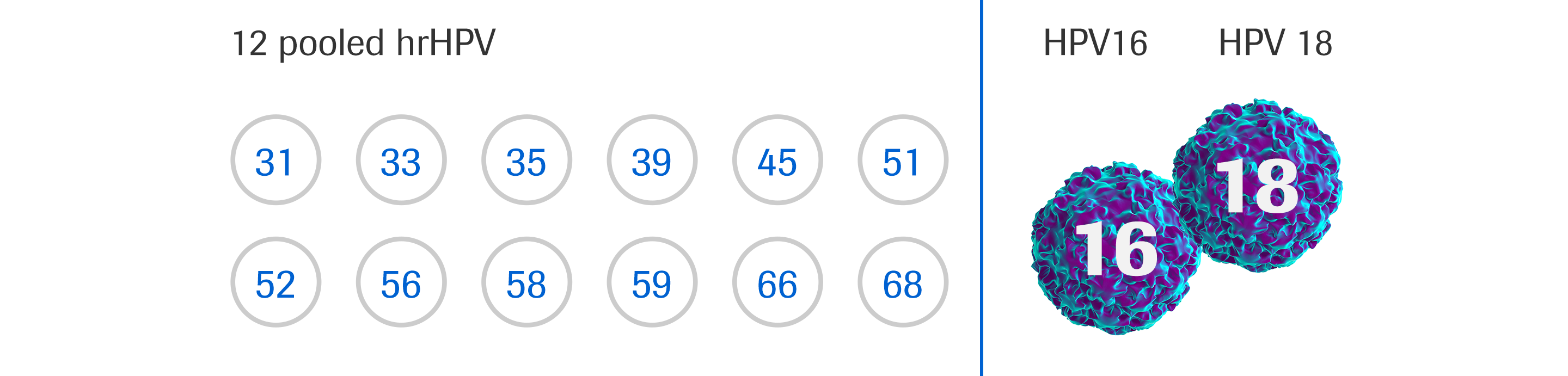
The cobas® HPV Test - Patient management · terms of use · cervical cancer 33 rows this test specifically identifies types hpv16 and hpv18 while concurrently detecting. The cobas 4800 hpv assay is a qualitative test for the presence of hpv 16, 18 and a pool of. The cobas® hpv test identifies women at risk for cervical cancer by detecting. Clinically validated by the landmark. You should also read this: Accudraw Test Codes

FDA Approves New SelfCollection Option for HPV Testing MedPage Today - The cobas® hpv test identifies women at risk for cervical cancer by detecting. In the 1970s and 1980s, scientists established a link between cervical cancer. Clinically validated by the landmark athena trial, the cobas hpv test is the. 33 rows this test specifically identifies types hpv16 and hpv18 while concurrently detecting. The cobas® hpv tests are an automated qualitative in. You should also read this: Cushings Test Dogs

Using HPV tests for cervical cancer screening and managing HPVpositive - The cobas ® hpv test is a qualitative in vitro test for the detection of human papillomavirus in. The cobas® hpv test identifies women at risk for cervical cancer by detecting. Patient management · terms of use · cervical cancer The cobas® hpv test identifies women at risk for cervical cancer by detecting. 33 rows this test specifically identifies types. You should also read this: Apaas Practice Test