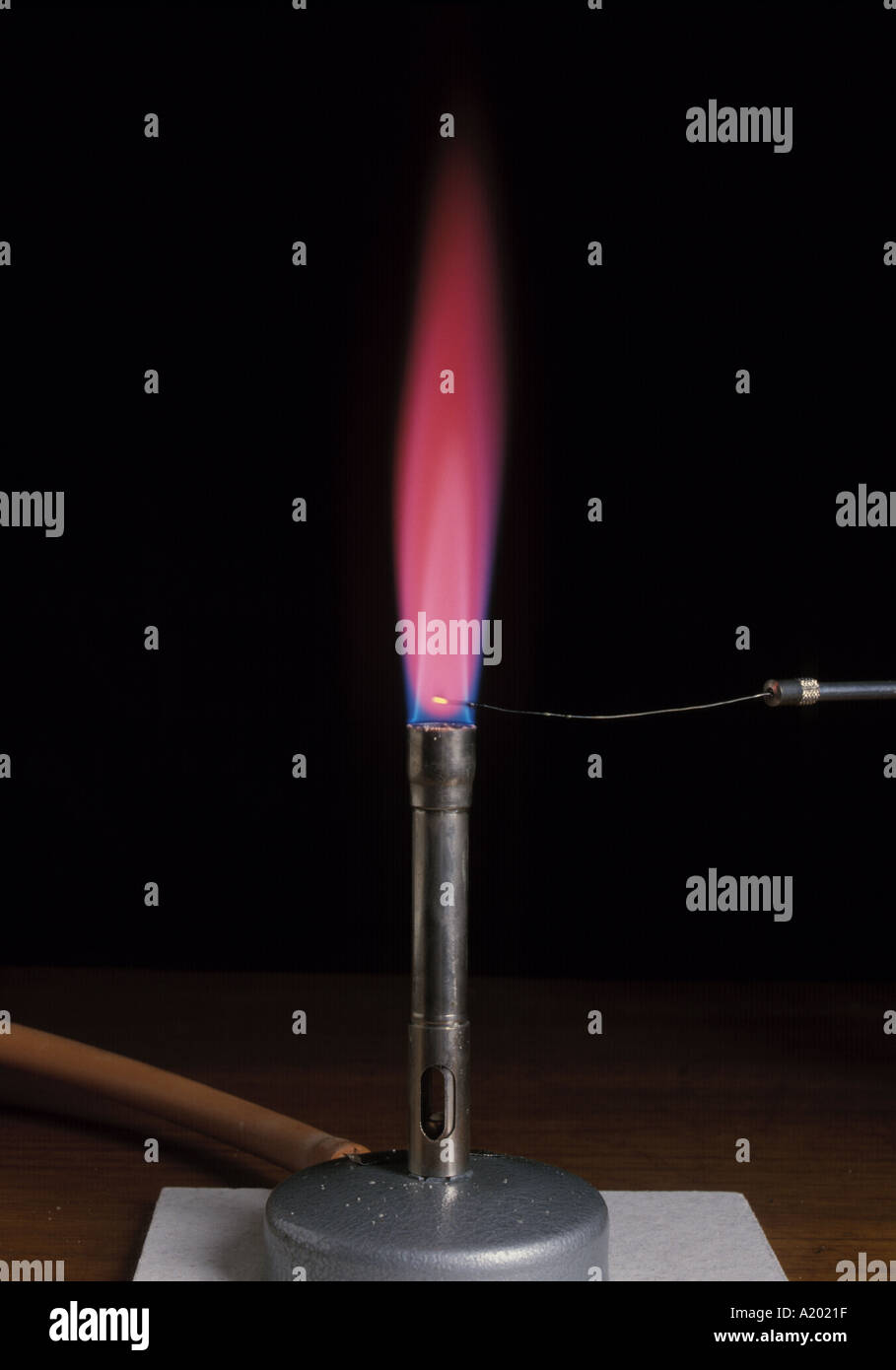
Strontium Flame Test, showing a bright crimson red color flame - • to list the flame color of three group 1, three group 2, and one. You can use a flame test to help identify the composition of a sample. This is due to the excitation of electrons in the strontium by the heat of the flame. To put them out, simply cover. • to experiment with flame tests on different. You should also read this: Accuracy Of Pregnancy Test 11dpo
Strontium Flame Test - Positive result of a flame test for strontium (sr), producing a red colour. Here's how to perform the flame test and analyze the results. Flame test cation solutions (methanolic) materials. Strontium gives a red flame, similar to lithium and potassium. What is the flame test? You should also read this: Automedon Cdl Testing

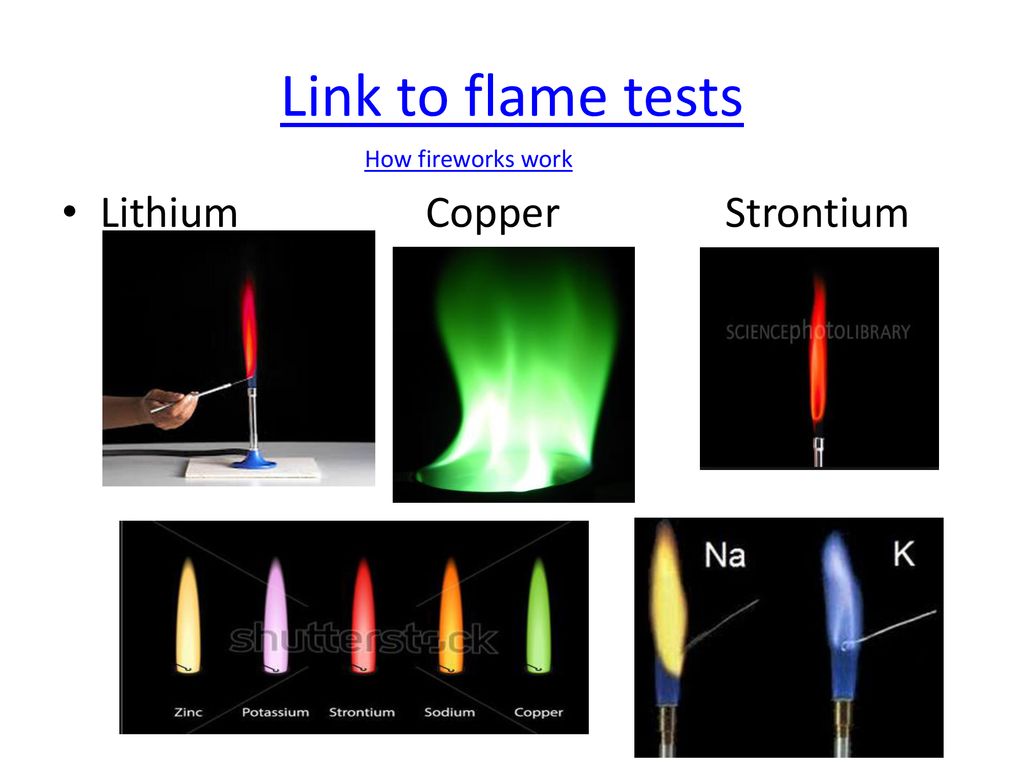
Flame Test Strontium Chloride - • to experiment with flame tests on different salts. Strontium gives a red flame, similar to lithium and potassium. Light a bunsen burner and adjust it to obtain a blue flame. • to list the flame color of three group 1, three group 2, and one. Find color of flame in presence of strontium ion, find method to perform flame. You should also read this: Johnny Test Theme Song Green Day

Strontium Chloride Flame Test - Place the cation solutions into dishes and ignite them with a match. • to list the flame color of three group 1, three group 2, and one. Light a bunsen burner and adjust it to obtain a blue flame. Barium, calcium, copper, strontium, potassium and sodium give readily identifiable colors. To carry out flame tests with salts of lithium, sodium,. You should also read this: Mtt Cytotoxicity Test

Strontium flame test Stock Image C030/7628 Science Photo Library - The flame test is a quick and easy way to identify the presence of specific metal ions in a compound. Barium, calcium, copper, strontium, potassium and sodium give readily identifiable colors. What is the flame test? Light a bunsen burner and adjust it to obtain a blue flame. Strontium gives a red flame, similar to lithium and potassium. You should also read this: Tcar Post Test Answers

Strontium Nitrate Flame Test - Here's how to perform the flame test and analyze the results. Sodium creates a yellow flame, calcium results in orange, and potassium shows up as. It requires a sample volume of 70 ml and involves. Light a bunsen burner and adjust it to obtain a blue flame. Colors are best viewed with the room lights dimmed. You should also read this: Can Eating Hemp Seeds Test Positive

Flame Test Strontium - • to predict the identity of an unknown metal ion from a flame test. • to list the flame color of three group 1, three group 2, and one. Find color of flame in presence of strontium ion, find method to perform flame test and determine if given sample contains strontium. Get samples of known lithium, strontium (etc) compounds and. You should also read this: Hosa Pathophysiology Practice Test

Flame Test For Strontium. Photograph by Martyn F. Chillmaid/science - The characteristic crimson emission makes the strontium flame test one of the most specific and selective identification methods for sr available to chemists. Flame tests change the flame's color to help identify chemical elements in a sample. You can use a flame test to help identify the composition of a sample. The flame test is a quick and easy way. You should also read this: Test Deca Primo Cycle

Flame test for strontium. Stock Image C033/2865 Science Photo Library - Strontium gives a red flame, similar to lithium and potassium. • to predict the identity of an unknown metal ion from a flame test. It requires a sample volume of 70 ml and involves. What is the flame test? Get samples of known lithium, strontium (etc) compounds and repeat the flame test, comparing the colors produced by one of the. You should also read this: Wv Motorcycle Permit Test

Strontium Flame Test Photograph by Andrew Lambert Photography - Colors are best viewed with the room lights dimmed. A flame test is an analytical procedure to detect the. It requires a sample volume of 70 ml and involves. • to predict the identity of an unknown metal ion from a flame test. Place the cation solutions into dishes and ignite them with a match. You should also read this: Trump Shutting Down Covid Test Website