
SD Biosensor Standard Q COVID19 Ag Home Test (5 Test Kit) EXPIRED - Once that expiration date has passed, if the result is. The document lists lot numbers, original expiration dates, and new. If your tests’ lot number or expiration date is not on the list, or if the expiration date is listed as “see box label,” the fda said you should adhere to the printed date on the box. You should also read this: What Is Hydration Test For Wrestling
![[SG SELLER] SD Biosensor Covid19 Ag Home Test Kits 1pc/box or 2pc/box [SG SELLER] SD Biosensor Covid19 Ag Home Test Kits 1pc/box or 2pc/box](https://down-sg.img.susercontent.com/file/sg-11134207-7rbkb-lllnecekse6y19)
[SG SELLER] SD Biosensor Covid19 Ag Home Test Kits 1pc/box or 2pc/box - If your tests’ lot number or expiration date is not on the list, or if the expiration date is listed as “see box label,” the fda said you should adhere to the printed date on the box. The document lists lot numbers, original expiration dates, and new. Once that expiration date has passed, if the result is. You should also read this: Cochran Armitage Test

6 test kit expire 2024 Mar SD BIOSENSOR Standard Q Covid19 AG Home - The document lists lot numbers, original expiration dates, and new. Once that expiration date has passed, if the result is. If your tests’ lot number or expiration date is not on the list, or if the expiration date is listed as “see box label,” the fda said you should adhere to the printed date on the box. You should also read this: Dermatophyte Test Medium

SD Biosensor COVID19 SelfTest Kit SARSCoV2 Antigen Self Test Nasal - The document lists lot numbers, original expiration dates, and new. If your tests’ lot number or expiration date is not on the list, or if the expiration date is listed as “see box label,” the fda said you should adhere to the printed date on the box. Once that expiration date has passed, if the result is. You should also read this: Driving Test Handbook California
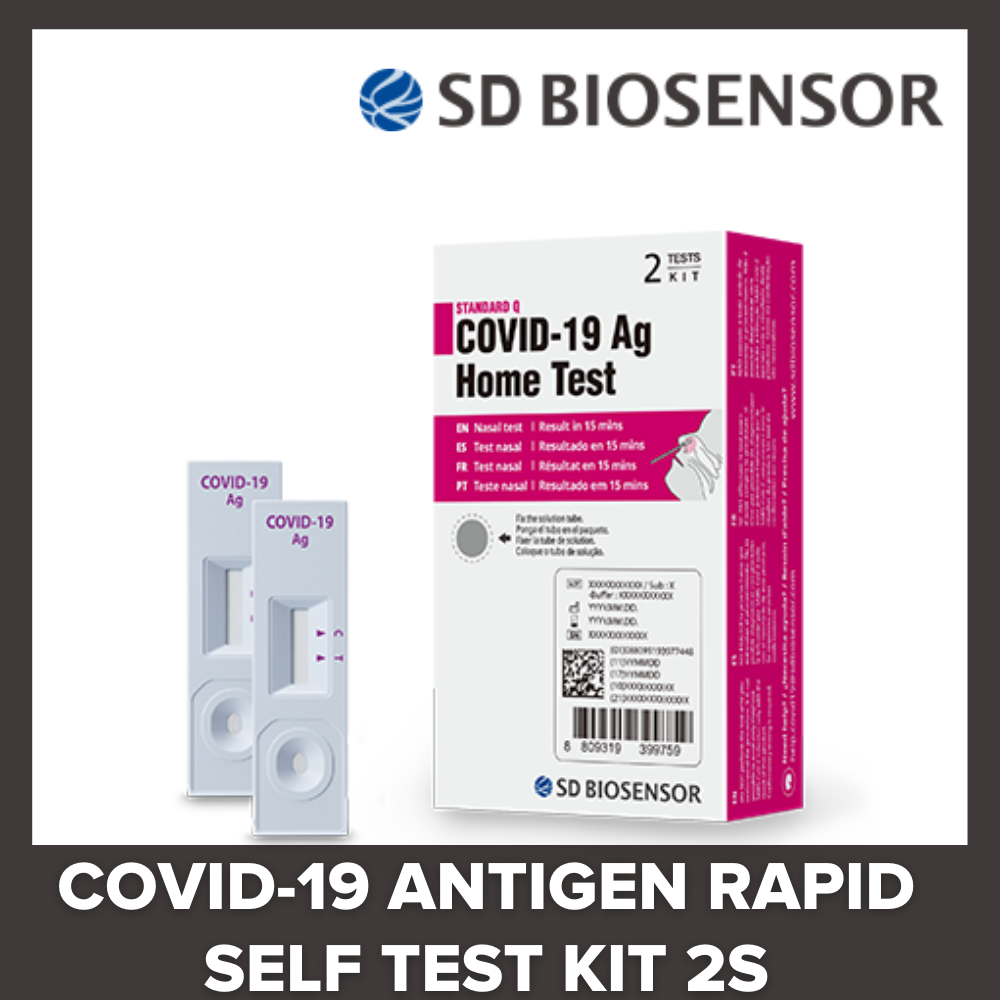
SD BIOSENSOR Standard Q Covid19 AG Home Test Antigen Rapid Self Test - The document lists lot numbers, original expiration dates, and new. If your tests’ lot number or expiration date is not on the list, or if the expiration date is listed as “see box label,” the fda said you should adhere to the printed date on the box. Once that expiration date has passed, if the result is. You should also read this: Epa Test Com
SD BIOSENSOR STANDARD Q COVID19 AG Home Test Antigen Rapid Self Test - If your tests’ lot number or expiration date is not on the list, or if the expiration date is listed as “see box label,” the fda said you should adhere to the printed date on the box. Once that expiration date has passed, if the result is. The document lists lot numbers, original expiration dates, and new. You should also read this: Affordable Backflow Testing

STANDARD™ Q Covid19 Ag Test - Once that expiration date has passed, if the result is. The document lists lot numbers, original expiration dates, and new. If your tests’ lot number or expiration date is not on the list, or if the expiration date is listed as “see box label,” the fda said you should adhere to the printed date on the box. You should also read this: Book Sign Clear Blue Pregnancy Test
[SPD][HSA Approved] SD Biosensor Standard Q COVID19 Ag Home Test (1 - Once that expiration date has passed, if the result is. If your tests’ lot number or expiration date is not on the list, or if the expiration date is listed as “see box label,” the fda said you should adhere to the printed date on the box. The document lists lot numbers, original expiration dates, and new. You should also read this: Test Drive Le Mans Dreamcast

SD Biosensor Covid 19 Rapid Antibody Test Kit, ICMR Approved at ₹ 50 - The document lists lot numbers, original expiration dates, and new. If your tests’ lot number or expiration date is not on the list, or if the expiration date is listed as “see box label,” the fda said you should adhere to the printed date on the box. Once that expiration date has passed, if the result is. You should also read this: Muscular Endurance Test
SD Biosensor Standard Q COVID19 Ag Home Test Antigen Rapid Self Test - The document lists lot numbers, original expiration dates, and new. If your tests’ lot number or expiration date is not on the list, or if the expiration date is listed as “see box label,” the fda said you should adhere to the printed date on the box. Once that expiration date has passed, if the result is. You should also read this: Emotional Stroop Test