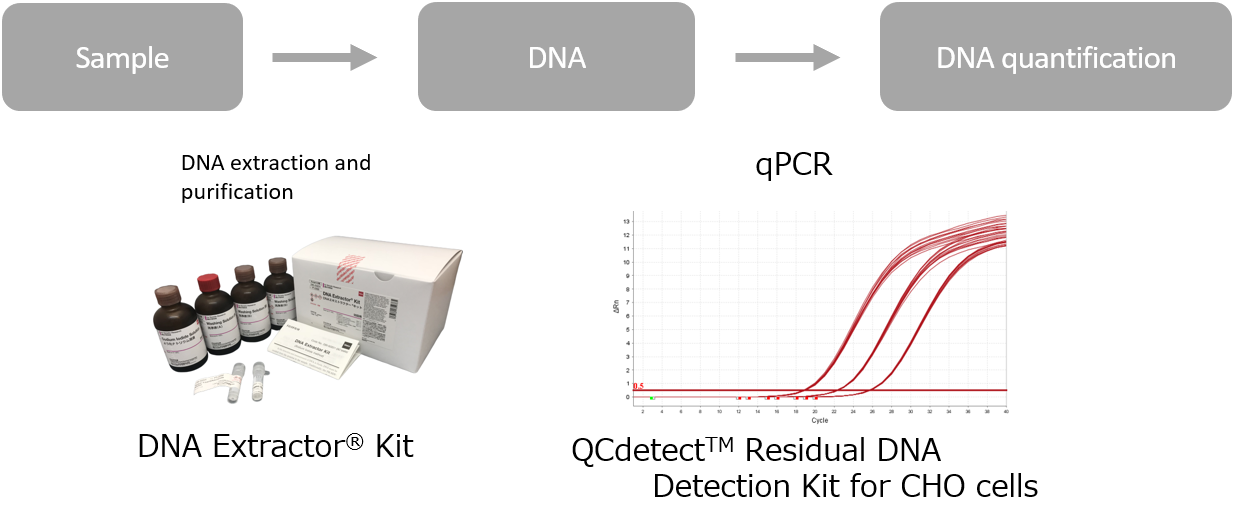
QCdetect™ Residual DNA Detection Kit for CHO cells・29485201[Detail - Levels of host cell dna needs to be monitored during process development and validation. Previous research has shown that ctdna tests accurately trace the progression of colorectal and breast. Dna testing is the most conclusive way of determining paternity or maternity in the eyes of the law. Learn to meet regulatory standards. Usp’s webinar provides an overview of usp general. You should also read this: Any Lab Test Now Boerne

Detection of residual host cell DNA by dPCR with MiQuant® - Eurofins is the industry leader in residual dna testing. Performance characteristics may not apply in tumor. By validating clearance during process validation or by monitoring residual dna levels by routine testing of. The main assays for residual dna are. How mrd testing can impact your care. You should also read this: Msk Impact Testing
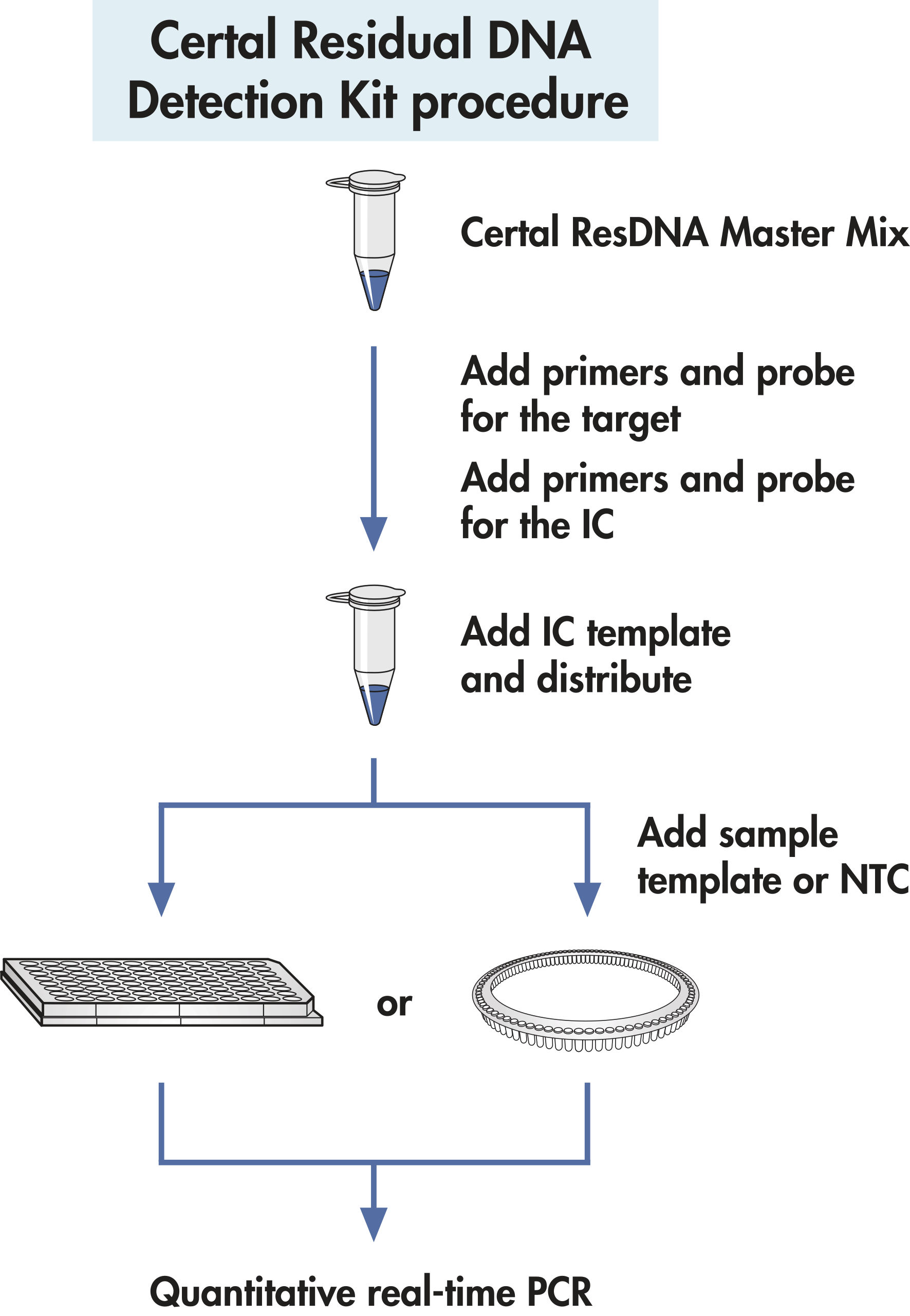
Certal Residual DNA Detection Kits - Eurofins is the industry leader in residual dna testing. The main assays for residual dna are. Open until 9:00 pma bbb rating, since 19953,500+ u.s. Established in 1998, the university of chicago genetic services laboratories has one of the most comprehensive menus of genetic tests available for inherited disorders, including neurological. One can address residual dna in biopharmaceutical processes in. You should also read this: Dependency Disorder Test
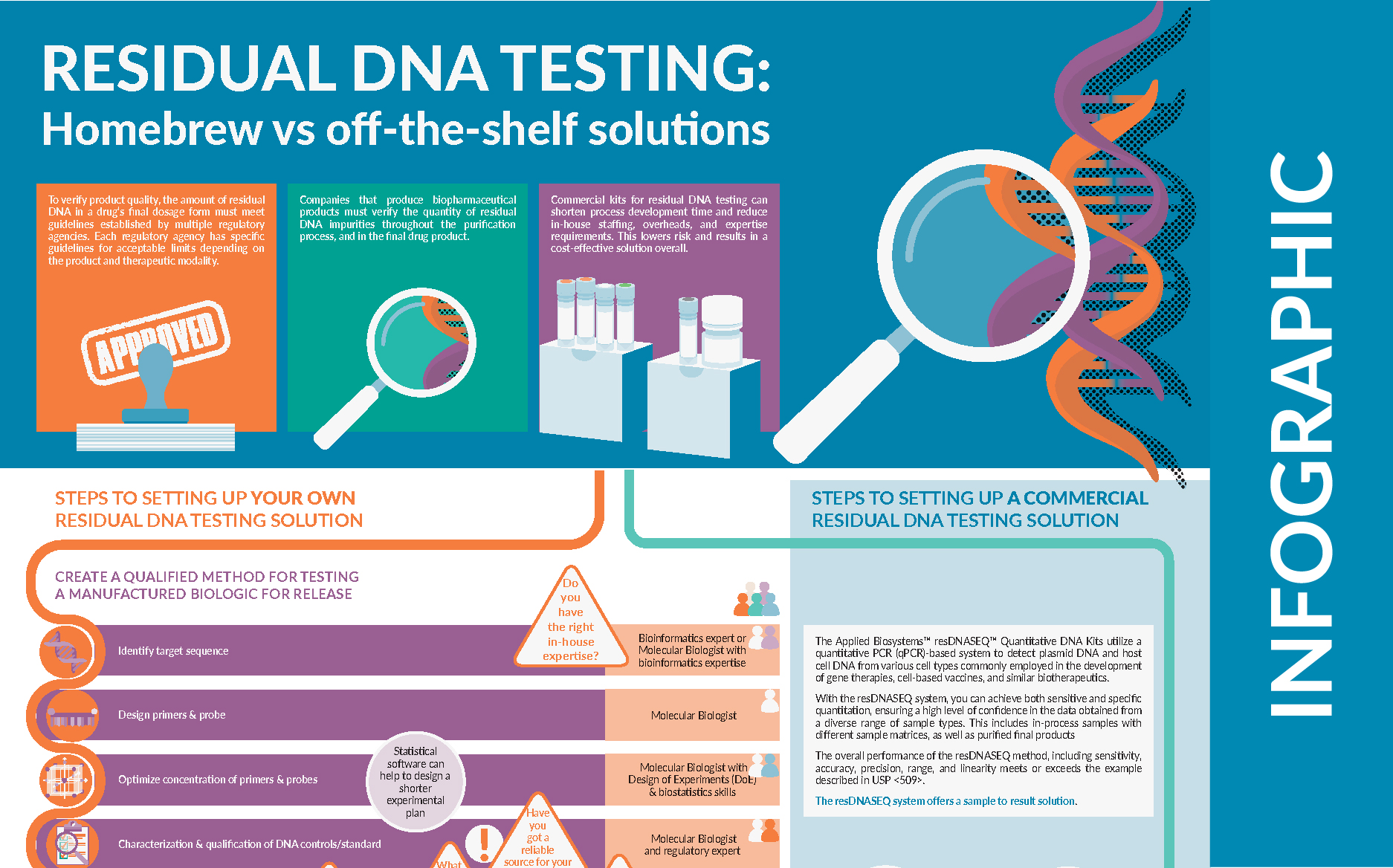
BioInsights Residual DNA testing Homebrew vs offtheshelf solutions - Performance characteristics may not apply in tumor. We offer our clients in chicago and throughout north america an unparalleled level of. Genetrack biolabs has been providing the highest standards in laboratory testing for over 20 years. However, it is not utilised in melanoma management. Residual host cell dna must be minimized as much as possible; You should also read this: Asbestos Testing Clearwater
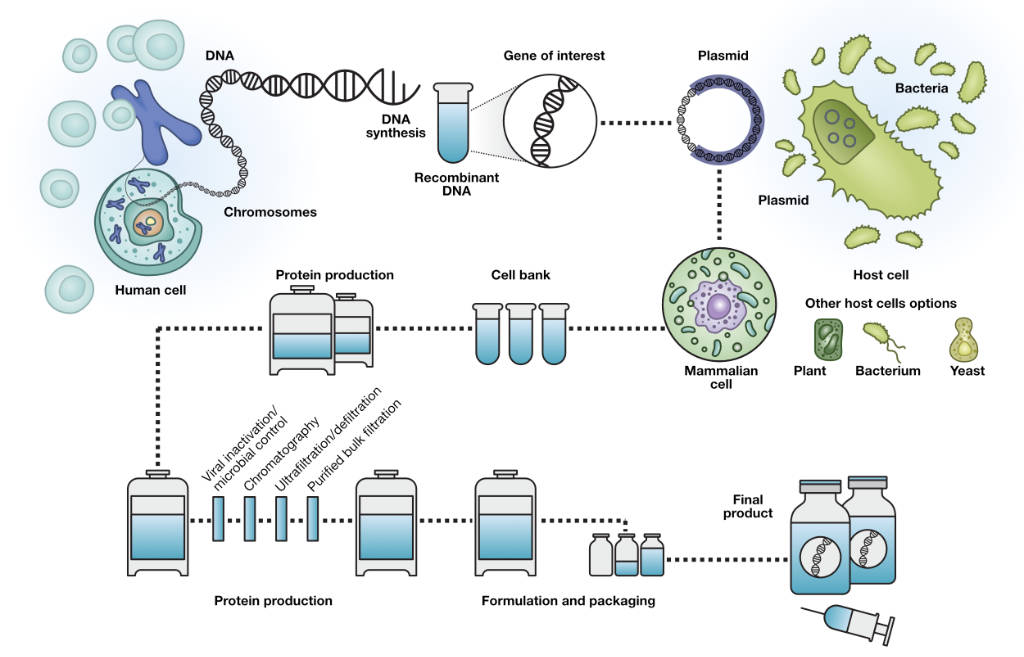
Single Use Technology Residual Analysis - Measure levels of residual host dna with the applied biosystems resdnaseq host cell residual dna quantitation system. Residual dna testing, also known as host cell dna analysis, is an essential quality control measure used to detect and quantify trace amounts of dna from host cells that may. Performance characteristics may not apply in tumor. Genetrack biolabs has been providing the. You should also read this: Beat Etg Test

Genomic approaches to cancer and minimal residual disease detection - The new test analyzes rna molecules in the bloodstream. Exas), a leading provider of cancer screening and diagnostic tests, today announced the launch of oncodetect™—a new test designed to detect. Established in 1998, the university of chicago genetic services laboratories has one of the most comprehensive menus of genetic tests available for inherited disorders, including neurological. Usp’s webinar provides an. You should also read this: Fatal Flaw Test

Residual DNA CHO Kit, Kit for determination of residual DNA CHO - By validating clearance during process validation or by monitoring residual dna levels by routine testing of. The following method is suitable for measurement of residual host cell dna in recombinant therapeutic products produced in either escherichia coli (e. Learn to meet regulatory standards. Dna testing is the most conclusive way of determining paternity or maternity in the eyes of the. You should also read this: Florida Drug And Alcohol Test Answers
resDNASEQ™ Human Residual DNA Quantitation Kit - Coli or chinese hamster ovary. The following method is suitable for measurement of residual host cell dna in recombinant therapeutic products produced in either escherichia coli (e. Residual host cell dna must be minimized as much as possible; The mutated dna spills into surrounding blood as the cells break down. Usp general chapter provides a validated method suitable for measurement. You should also read this: Mmp 9 Blood Test
'Applied Biosystems' resDNASEQ™ Host Cell Residual DNA Quantitation - One can address residual dna in biopharmaceutical processes in two ways: Genetrack biolabs has been providing the highest standards in laboratory testing for over 20 years. The main assays for residual dna are. Previous research has shown that ctdna tests accurately trace the progression of colorectal and breast. Establishing appropriate host cell residual dna assays can help monitor the production. You should also read this: How To Test An Outboard Ignition Coil

Residual DNA Testing Charles River - The mutated dna spills into surrounding blood as the cells break down. Residual host cell dna must be minimized as much as possible; The following method is suitable for measurement of residual host cell dna in recombinant therapeutic products produced in either escherichia coli (e. Eurofins is the industry leader in residual dna testing. We offer our clients in chicago. You should also read this: Arcane Personality Test