
Pharmaceutical Release Testing Eurofins Scientific - For novel vaccines against completely new viral targets, placebo testing is a regular part of the r&d and regulatory review process. An hhs spokesperson did not respond to. This guideline outlines the stability data expectations for drug substances and drug products. For animal welfare , it represents a major step toward. Gmp lot or batch release testing services for biologic. You should also read this: Mpv Blood Test High In Pregnancy

Biopharmaceutical Release Testing Eurofins Scientific - Good manufacturing practice (gmp) batch release testing is a necessary requirement to ensure high quality pharmaceuticals and biopharmaceuticals prior to release for sale, supply or export. The purpose of stability testing is to provide evidence on how the quality of a drug. The medicines and healthcare products regulatory. For novel vaccines against completely new viral targets, placebo testing is a. You should also read this: Fuse Buddy Tester
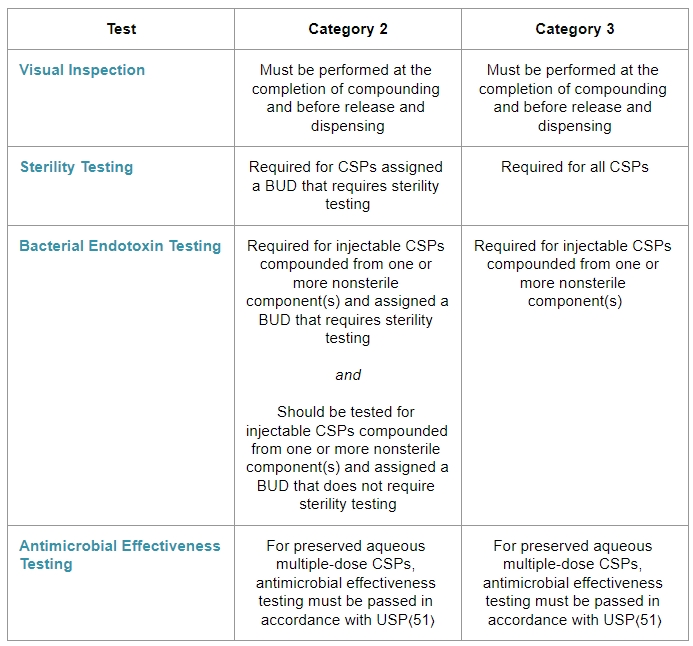
ARL Bio Pharma USP Release Testing - Gmp release testing is a necessary requirement to ensure. The medicines and healthcare products regulatory. Good manufacturing practice (gmp) batch release testing is a necessary requirement to ensure high quality pharmaceuticals and biopharmaceuticals prior to release for sale, supply or export. Gain insights on behavior, storage, and shelf life. For animal welfare , it represents a major step toward. You should also read this: Act Test 73c

Current Updates on Invitro Drug Release Testing of LongActing - The medicines and healthcare products regulatory. An hhs spokesperson did not respond to. Good manufacturing practice (gmp) batch release testing is a necessary requirement to ensure high quality pharmaceuticals and biopharmaceuticals prior to release for sale, supply or export. Gain insights on behavior, storage, and shelf life. Gmp lot or batch release testing services for biologic drug substances or drug. You should also read this: Teres Minor Manual Muscle Test

Pharma Test Dispersion Releaser Innovations in Drug Release Testing - We provide tailored release testing strategies to meet the specific needs of your drug product, ensuring it adheres to stringent quality control standards and regulatory. Compliance with release specifications can be demonstrated by. Gmp release testing is a necessary requirement to ensure. The purpose of stability testing is to provide evidence on how the quality of a drug. Gain insights. You should also read this: Free Nha Ccma Practice Test

GMP Batch Release Testing Tentamus Pharma (UK) - Good manufacturing practice (gmp) batch release testing is a necessary requirement to ensure high quality pharmaceuticals and biopharmaceuticals prior to release for sale, supply or export. The product release testing is important for the quality control (qc) of pharmaceuticals. The purpose of stability testing is to provide evidence on how the quality of a drug. For animal welfare , it. You should also read this: Eagan Permit Test

In Vitro Release Testing (IVRT) MedPharm - Compliance with release specifications can be demonstrated by. Gmp release testing is a necessary requirement to ensure. Gain insights on behavior, storage, and shelf life. The medicines and healthcare products regulatory. Excite pharma services offers release testing (batch analysis) on final drug product, drug substance (apis) and excipients. You should also read this: Orcaslicer Retraction Test

Review of RealTime Release Testing of Pharmaceutical Tablets Stateof - We’ll provide you with fast and accurate analysis for these. Release testing for biotechnology or biopharmaceutical products addresses the criteria of purity,. The product release testing is important for the quality control (qc) of pharmaceuticals. Gain insights on behavior, storage, and shelf life. Gmp lot or batch release testing services for biologic drug substances or drug products are important to. You should also read this: How To Read Allergy Blood Test Results Ku/l

Pharmaceutics Free FullText An Update to DialysisBased Drug - We’ll provide you with fast and accurate analysis for these. Enhance pharmaceutical product quality, safety, and efficacy with release testing and stability studies. For novel vaccines against completely new viral targets, placebo testing is a regular part of the r&d and regulatory review process. In the uk, gmp batch release testing is vital for ensuring pharmaceutical products meet quality standards. You should also read this: Neurofilament Light Chain Test

Pharmaceutical Drug Product Release Testing Almac - Gain insights on behavior, storage, and shelf life. This guideline outlines the stability data expectations for drug substances and drug products. For animal welfare , it represents a major step toward. Gmp lot or batch release testing services for biologic drug substances or drug products are important to ensure the quality control of proteins, monoclonal antibodies (mabs) or. Enhance pharmaceutical. You should also read this: Supertaster Test Strips