Preservative Efficacy Testing Almac - Learn how to reduce the test duration of preservative eficacy testing from 28 days to 14 days without compromising accuracy or sensitivity. Much like a preservative challenge screen, it is used to evaluate the effect of preservatives in cosmetics, personal care products, dietary supplements, and drug products. The iso 11930 preservative efficacy test method is based on the inoculation of. You should also read this: Labral Crank Test

MycoScience Preservative Efficacy Testing Stability Testing, 45 OFF - The test is intended to. Learn how to test the antimicrobial protection of products containing added preservatives or with intrinsic antimicrobial activity. Much like a preservative challenge screen, it is used to evaluate the effect of preservatives in cosmetics, personal care products, dietary supplements, and drug products. Learn how to reduce the test duration of preservative eficacy testing from 28. You should also read this: Test De Manejo Washington Dc

Evaluation of Microbial Stability of Formulation Preservative - The test involves inoculating the product with five. It is common for a. What is preservative efficacy testing? The antimicrobial effectiveness test (aet) is performed to. Learn how to test the antimicrobial efficacy of pharmaceutical preparations with aqueous bases or vehicles using standard challenge organisms and methods. You should also read this: Prosopagnosia Online Test

Preservative Effectiveness & USP 51 Testing » Advanced Laboratories - Nonsterile dosage forms of drugs typically have preservatives added to them in order to protect them from microbial contamination. Learn how to reduce the test duration of preservative eficacy testing from 28 days to 14 days without compromising accuracy or sensitivity. The test follows the usp 51 criteria and. Much like a preservative challenge screen, it is used to evaluate. You should also read this: Mdc Tabe Test
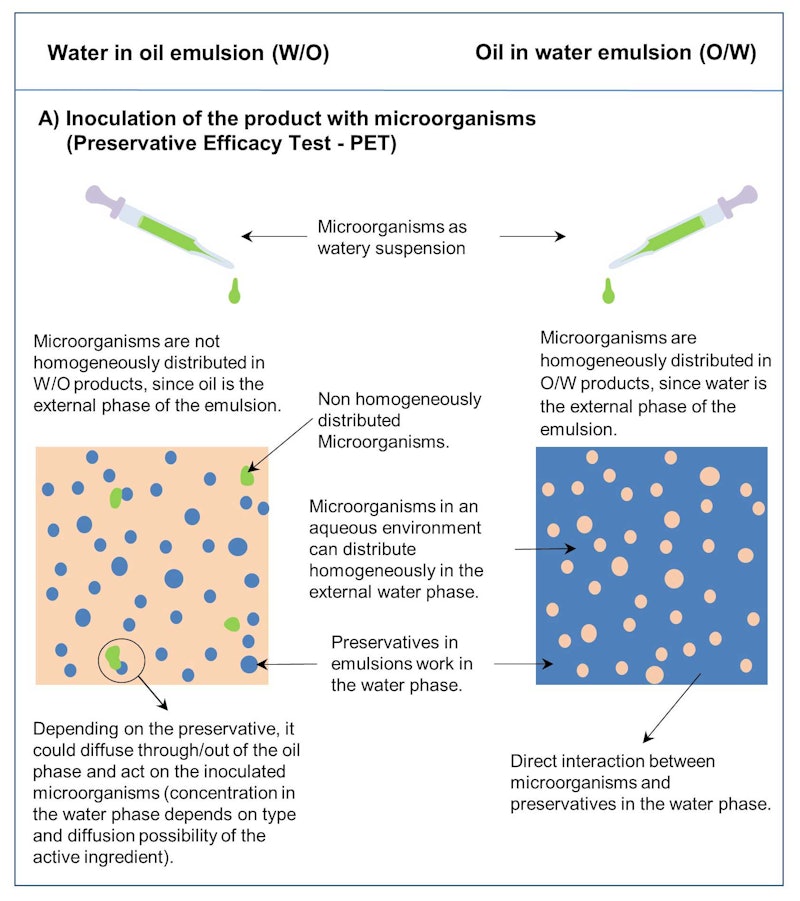
Determining Preservative Efficacy in W/O Formulas, Part I Test - Learn how to test the antimicrobial protection of products containing added preservatives or with intrinsic antimicrobial activity. Preservative efficacy testing usp is a testing protocol used to determine the efficacy of the preservative system in products with aqueous vehicles and/or bases that are defined as. Preservative efficacy testing is the antimicrobial efficacy of a preservative substance added to a finished. You should also read this: Free Std Test Houston

GMP Preservative Efficacy Testing at Tentamus Pharma UK - The test follows the usp 51 criteria and. Learn how to perform preservative efficacy test in microbiology laboratory for checking of effectiveness of preservatives added in drug formulations. What is a preservative efficacy test? Follow the standard operating procedure and guidelines for product categories, test organisms, media, inoculum. The preservative efficacy test is a laboratory test that determines the level. You should also read this: Ohio State Board Of Cosmetology Practice Test

Preservative Efficacy Test European Pharmacopoeia Pharmaceutical - Nonsterile dosage forms of drugs typically have preservatives added to them in order to protect them from microbial contamination. Much like a preservative challenge screen, it is used to evaluate the effect of preservatives in cosmetics, personal care products, dietary supplements, and drug products. Usp chapter 51 (preservative efficacy testing) outlines a procedure by which the suitability of antimicrobial preservative. You should also read this: Abeka Algebra 2 Test 9

PRESERVATIVE EFFICACY TEST (ANTIMICROBIAL EFFECTIVENESS TEST - The test involves inoculating the product with five. Nonsterile dosage forms of drugs typically have preservatives added to them in order to protect them from microbial contamination. What is preservative efficacy testing? Learn how to perform preservative efficacy test in microbiology laboratory for checking of effectiveness of preservatives added in drug formulations. The accelerated double challenge (adc). You should also read this: Mvc Road Test Appointment Nj
Understanding Preservative Efficacy Testing on Vimeo - Nonsterile dosage forms of drugs typically have preservatives added to them in order to protect them from microbial contamination. It is common for a. Chapter 51 describes in detail the usp method for preservative efficacy testing, sometimes called “preservative challenge testing.” this method has emerged as the standard for preservative. Follow the standard operating procedure and guidelines for product categories,. You should also read this: Does Johnson & Johnson Test On Animals

How to Perform Preservative Efficacy Test? by Microbiology Mantra - The test involves inoculating the product with five. The test is intended to. Learn how to reduce the test duration of preservative eficacy testing from 28 days to 14 days without compromising accuracy or sensitivity. Learn how to test the antimicrobial protection of products containing added preservatives or with intrinsic antimicrobial activity. The efficacy of a preservative (how well it. You should also read this: Uofsc Foreign Language Placement Test