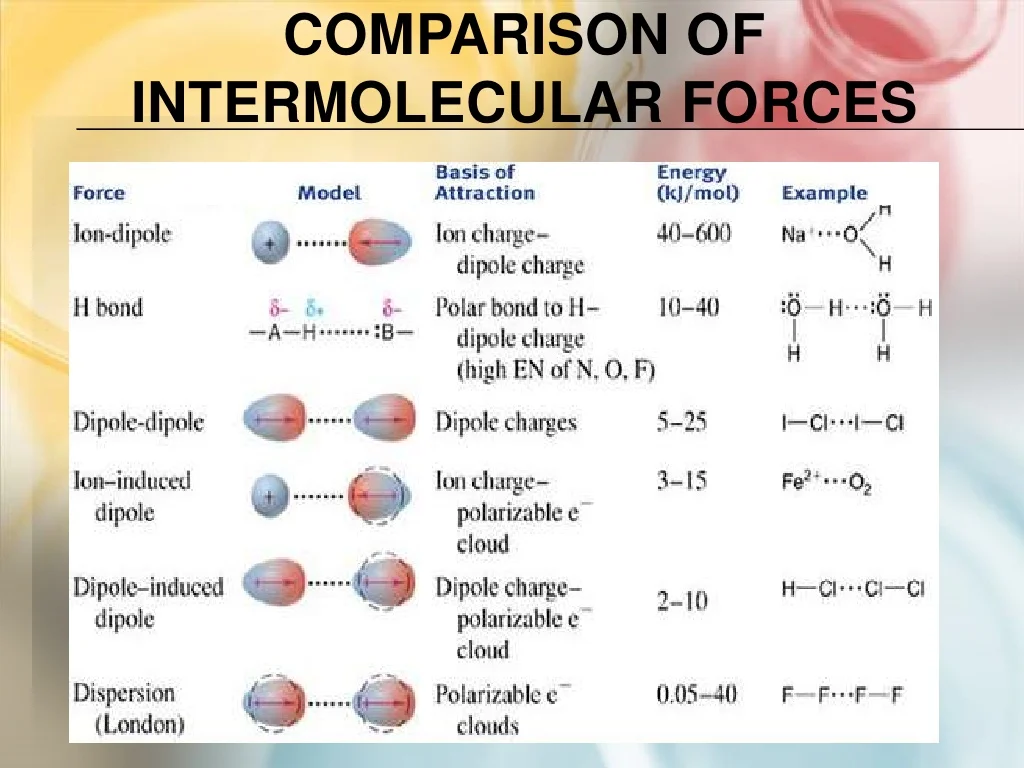
Intermolecular forces - What happens to the freezing point when particles are dissolved in a solvent? Hydrogen (h₂) and fluorine (f₂): Study with quizlet and memorize flashcards containing terms like molarity, mass percent, solubility and more. Na 3 po 4 calcium chloride: Based on their molecular structures, how does the boiling point of hf compare with the boiling points of h2 and f2? You should also read this: Lmsw Test Prep

B.Sc 1st semester Molecular Polarity and Weak Chemical Forces Important - Intermolecular forces and solubility post lab questions 1. Hydrogen (h₂) and fluorine (f₂): Dashboard | rapididentity post test: These molecules are nonpolar and primarily exhibit london dispersion forces, which are the weakest intermolecular forces. Study with quizlet and memorize flashcards containing terms. You should also read this: Equate Ovulation Test Results

Solved Draw molecularlevel representations of 4 M solutions - The solvent is the most concentrated component and. Quizlet has study tools to help you learn anything. Quizlet has study tools to help you learn anything. Study with quizlet and memorize flashcards containing terms like molarity, mass percent, solubility and more. A solution forms when two or more substances combine physically to yield a mixture that is homogeneous at the. You should also read this: San Clemente Dmv Driving Test Route

CHEMISTRY 101 Compare the solid, liquid and gas phases at the - Would carrying the barometer to the top of a mountain result in the level of mercury in the glass tube of a barometer at sea level. View unit 2 post test study guide.pdf from che physical c at denham springs high school. The solvent is the most concentrated component and. Study with quizlet and memorize flashcards containing terms. Study with. You should also read this: Are Dollar Tree Pregnancy Test Reliable
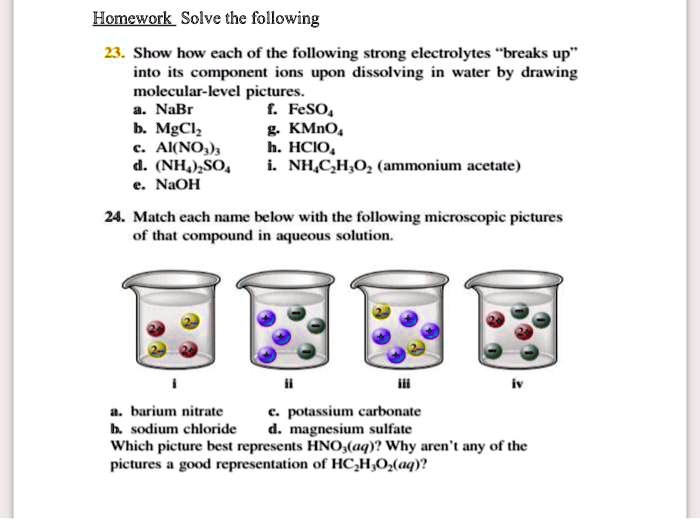
SOLVED Homawok Solve the following 3. Show how each of the following - Get instant answer verification, watch video solutions, and gain a deeper understanding of this. Study with quizlet and memorize flashcards containing terms. Now we consider the attractive forces between a solvent molecule and a. Quizlet has study tools to help you learn anything. Solubility and intermolecular forces with interactive practice questions. You should also read this: Tubular Ectasia Of The Rete Testes

The following pictures represent solutions at various stages in t - What happens to the freezing point when particles are dissolved in a solvent? Attractive forces between molecules in pure substances (solvents and solutes) were considered in the last chapter. Chemistry i, semester 2 unit 2: Get instant answer verification, watch video solutions, and gain a deeper understanding of this. Quizlet has study tools to help you learn anything. You should also read this: Troponin Test Tube Color
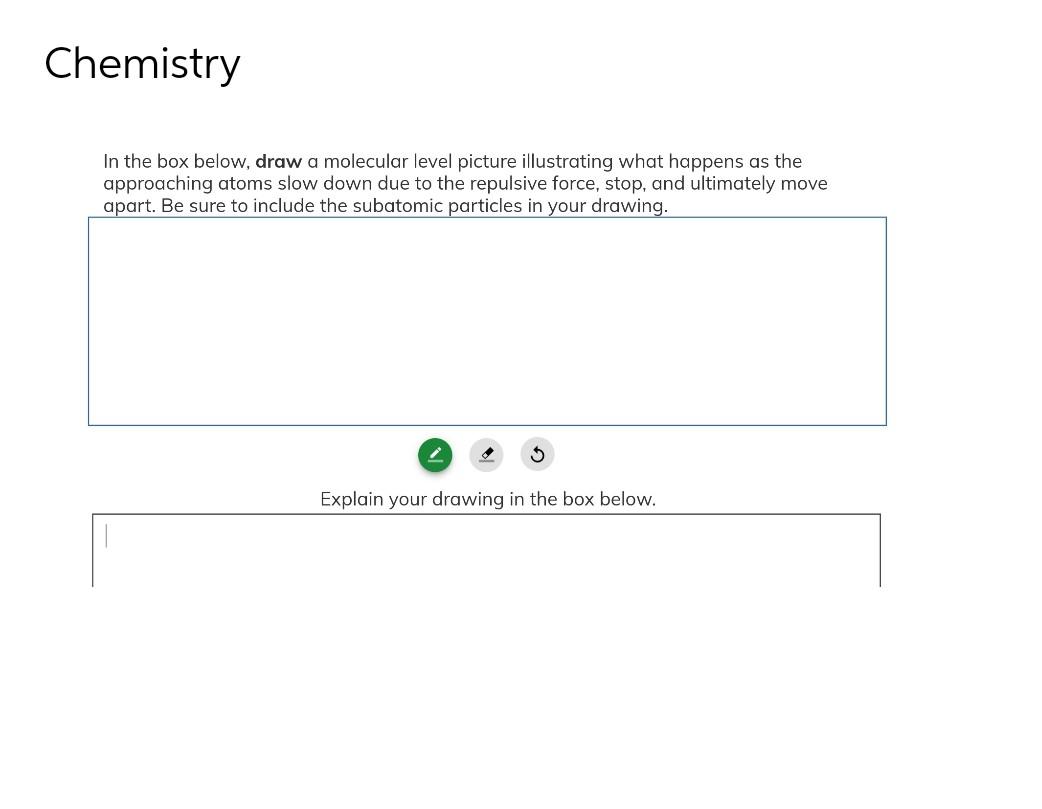
Solved Chemistry In the box below, draw a molecular level - Quizlet has study tools to help you learn anything. Would carrying the barometer to the top of a mountain result in the level of mercury in the glass tube of a barometer at sea level. Intermolecular forces and solubility post lab questions 1. Attractive forces between molecules in pure substances (solvents and solutes) were considered in the last chapter. Now. You should also read this: Are True Metrix Test Strips Compatible With Freestyle Libre

Draw molecularlevel pictures to differentiate betwee… SolvedLib - Solubility and intermolecular forces with interactive practice questions. The solvent is the most concentrated component and. Based on their molecular structures, how does the boiling point of hf compare with the boiling points of h2 and f2? Quizlet has study tools to help you learn anything. View unit 2 post test study guide.pdf from che physical c at denham springs. You should also read this: Piedmont Urgent Care Drug Test Reviews
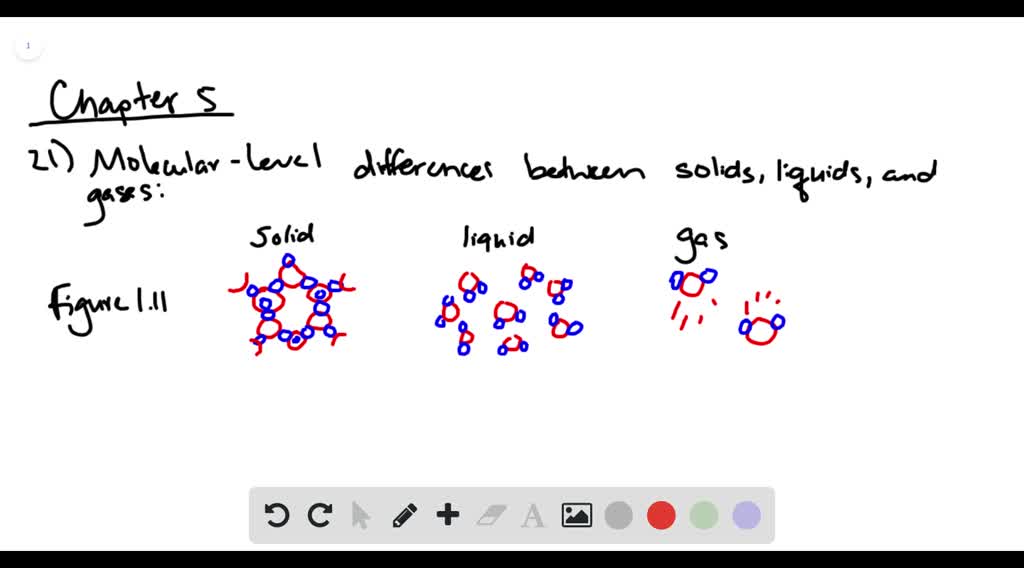
SOLVED Draw molecularlevel views that show the differences among - These molecules are nonpolar and primarily exhibit london dispersion forces, which are the weakest intermolecular forces. Post test molecular level forces and solutions question: Solubility and intermolecular forces with interactive practice questions. What happens to the freezing point when particles are dissolved in a solvent? A solution forms when two or more substances combine physically to yield a mixture that. You should also read this: Any Lab Test Now Chattanooga Tn
Unit 2 Post Test - Attractive forces between molecules in pure substances (solvents and solutes) were considered in the last chapter. Would carrying the barometer to the top of a mountain result in the level of mercury in the glass tube of a barometer at sea level. Dashboard | rapididentity post test: Hydrogen (h₂) and fluorine (f₂): Based on their molecular structures, how does the. You should also read this: Hvac Ase Practice Test