Your USP 797 Viable Sampling Questions Answered on Vimeo - What does usp say regarding media fills? During a media fill, technicians use microbiological growth media instead of a drug solution to assess whether the aseptic procedures performed are adequate to prevent contamination. The media fill test assesses the sterile technique of the trainee and related practices. Learn the basics of cleanroom design, risk levels, and sterile preparation. The test. You should also read this: What Is Criterion Referenced Test

MediaFill Testing Kits Hardy Diagnostics PP&P Magazine Pharmacy - During a media fill, technicians use microbiological growth media instead of a drug solution to assess whether the aseptic procedures performed are adequate to prevent contamination. The aseptic manipulation competency demonstration mandates the completion of a semiannual media fill test (mft) alongside concurrent surface test (st) and gloved fingertip. By adapting to the new usp media fill testing requirements, clinical. You should also read this: Ishihara Card Test

HardyVal™ Media Fill Test Kit Product Line for USP 797 YouTube - Proposed usp 797 guidelines state that if a compounding personnel fails a media fill test then they must successfully pass 3 additional tests prior to compounding. Find out the frequency, procedure, and. The media fill test assesses the sterile technique of the trainee and related practices. Initial & ongoing training and competency » training and competency. The united states pharmacopeia. You should also read this: Std Test Milwaukee
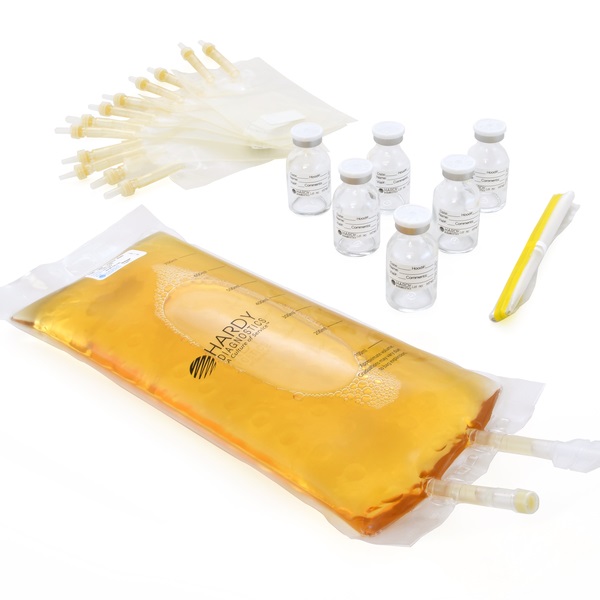
Hardy Diagnostics - During a media fill, technicians use microbiological growth media instead of a drug solution to assess whether the aseptic procedures performed are adequate to prevent contamination. By adapting to the new usp media fill testing requirements, clinical microbiologists can play a pivotal role in safeguarding patient safety and ensuring the highest standards of sterility. Find out the frequency, procedure, and.. You should also read this: Cpa Exam Test Score Release Dates
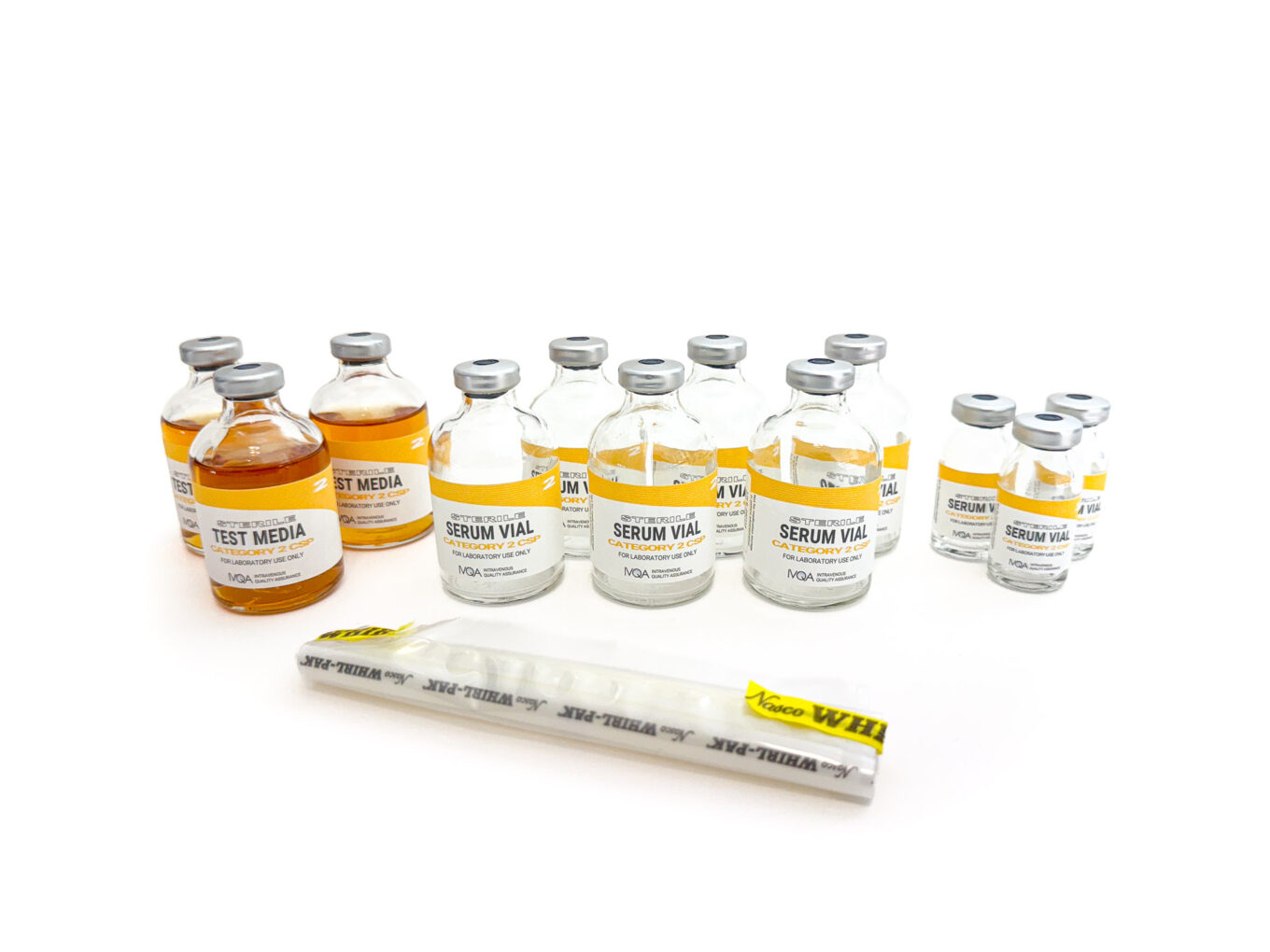
Category 2 CSP MediaFill Test Kit IVQA - By adapting to the new usp media fill testing requirements, clinical microbiologists can play a pivotal role in safeguarding patient safety and ensuring the highest standards of sterility. During a media fill, technicians use microbiological growth media instead of a drug solution to assess whether the aseptic procedures performed are adequate to prevent contamination. Learn the basics of cleanroom design,. You should also read this: Michigan Sos Driving Test
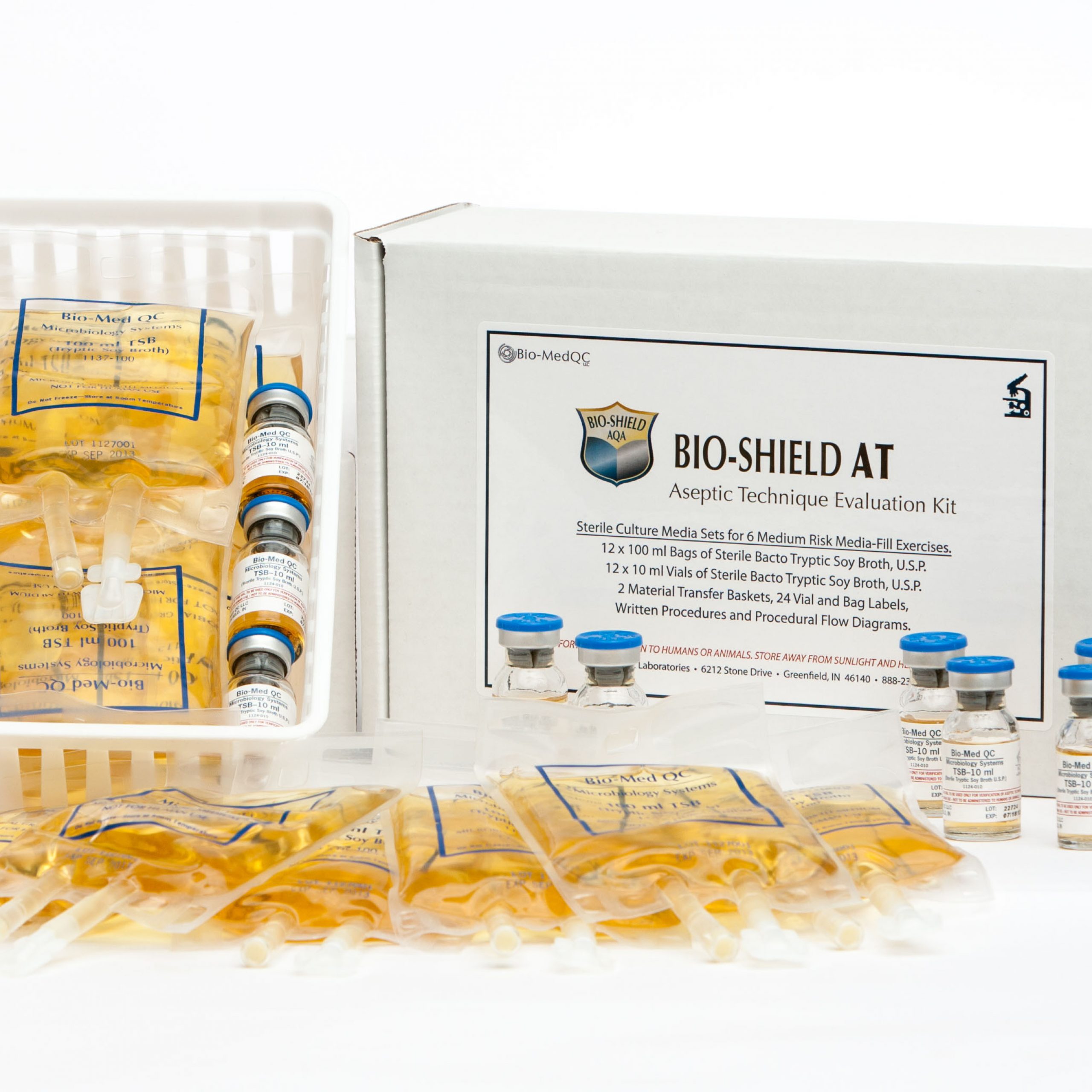
BioMed QC USP 797 Compliance Shop - Proposed usp 797 guidelines state that if a compounding personnel fails a media fill test then they must successfully pass 3 additional tests prior to compounding. The media fill test assesses the sterile technique of the trainee and related practices. A media fill test is required by united states pharmacopeia (usp) and state boards of pharmacy to prove that the. You should also read this: Electrolyte Testing Includes

2018 USP 797 Aerobiology Laboratory Associates - Learn the basics of cleanroom design, risk levels, and sterile preparation. The aseptic manipulation competency demonstration mandates the completion of a semiannual media fill test (mft) alongside concurrent surface test (st) and gloved fingertip. Initial & ongoing training and competency » training and competency. What does usp say regarding media fills? Proposed usp 797 guidelines state that if a compounding. You should also read this: Clear Blue Negative Pregnancy Test Results

The 2014 USP Chapter Compliance Survey October 2014 Cleanrooms - Initial & ongoing training and competency » training and competency. The media fill test assesses the sterile technique of the trainee and related practices. During a media fill, technicians use microbiological growth media instead of a drug solution to assess whether the aseptic procedures performed are adequate to prevent contamination. Learn the basics of cleanroom design, risk levels, and sterile. You should also read this: Gold Tester Gun Price

Sop For Media Fill Validation Pharmaceutical Guidelin vrogue.co - A media fill test is required by united states pharmacopeia (usp) and state boards of pharmacy to prove that the aseptic technique process can produce a sterile product without. During a media fill, technicians use microbiological growth media instead of a drug solution to assess whether the aseptic procedures performed are adequate to prevent contamination. Find out the frequency, procedure,. You should also read this: Ets The Official Guide To The Gre Revised General Test
MediumRisk Media Fill Test Kit Instructions on Vimeo - The aseptic manipulation competency demonstration mandates the completion of a semiannual media fill test (mft) alongside concurrent surface test (st) and gloved fingertip. Proposed usp 797 guidelines state that if a compounding personnel fails a media fill test then they must successfully pass 3 additional tests prior to compounding. By adapting to the new usp media fill testing requirements, clinical. You should also read this: Lenovo Vantage This Is A Test Notification