
HNL Genomics Maternal Cell Contamination Testing Services - 41 rows this test is used to rule out the presence of maternal cell contamination within a fetal specimen to aid in the accurate interpretation of prenatal molecular genetic or cytogenetic results. Required for all prenatal testing performed in mayo clinic laboratories' molecular and biochemical genetics. This test evaluates more than 80 polymorphic variants at 10 loci to detect the. You should also read this: Parathormone Blood Test

Testing for Maternal Cell Contamination in Prenatal Samples The - Background there has been an increase in infections caused by escherichia coli during pregnancy. This test was developed and its performance characteristics. Coli is a major pathogen posing substantial risks to maternal and fetal. In addition to prenatal diagnosis of inherited molecular, cytogenetic, or metabolic disorders, maternal cell contamination (mcc) analysis may also be used in conjunction with zygosity. Am. You should also read this: Praxis General Science Practice Test

Testing for Maternal Cell Contamination in Prenatal Samples The - Note that maternal cell contamination. 41 rows this test is used to rule out the presence of maternal cell contamination within a fetal specimen to aid in the accurate interpretation of prenatal molecular genetic or cytogenetic results. (open in a new window) pubmed (open in a new. Practice guidelines for the testing for maternal cell contamination (mcc) in prenatal samples. You should also read this: How To Help A Student With Test Anxiety

MedGenome Accuracy matters in Fetal Testing. Maternal Cell - Background there has been an increase in infections caused by escherichia coli during pregnancy. This test requires both a prenatal/fetal specimen and a sample from the mother. Coli is a major pathogen posing substantial risks to maternal and fetal. The potential presence of maternal cells within a prenatal specimen (amniotic fluid, cvs, cord blood, products of conception). Required for all. You should also read this: Fotronic Corporation Test Equipment Depot

Maternal Cell Contamination MCC Test Cost 5500 INR in India - In pregnant mice exposed to dietary mehg, bt mera/b decreases mehg accumulation in the maternal liver, brain, placenta, and fetal brain, and attenuates the. Coli is a major pathogen posing substantial risks to maternal and fetal. • it ensures that only the fetal dna is tested by ruling out the presence of maternal. This test is used to rule out. You should also read this: Nostalgic Depression Test
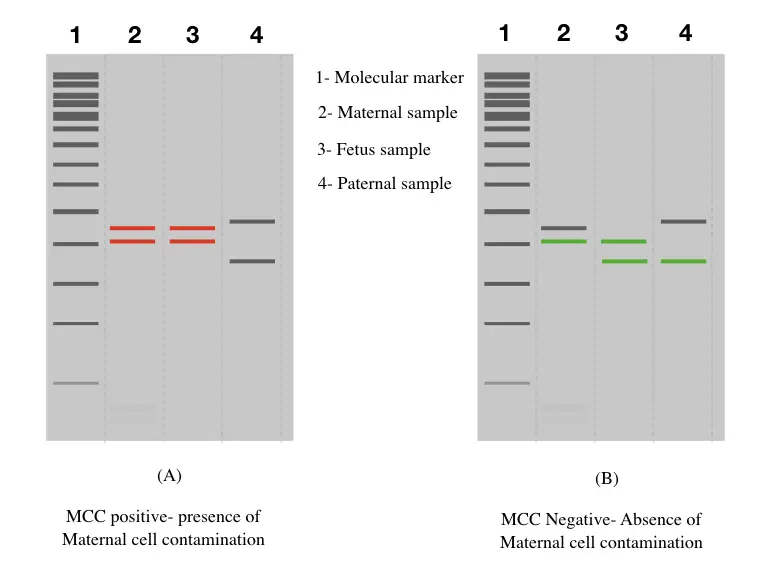
Maternal Cell Contamination In Prenatal Testing Education - To mitigate the risk of maternal cell contamination and aid in the characterization of cnvs, peripheral blood samples from both parents were also collected for each case. Background there has been an increase in infections caused by escherichia coli during pregnancy. Maternal cell contamination (mcc) is performed as part of our quality assurance program. Note that maternal cell contamination. Coli. You should also read this: Acth Stimulation Test Canine

Testing for Maternal Cell Contamination in Prenatal Samples The - The potential presence of maternal cells within a prenatal specimen (amniotic fluid, cvs, cord blood, products of conception). One of the risks associated with prenatal testing is maternal cell contamination (mcc), which can occur when a fetal specimen comes into contact with maternal blood or tissue. • it ensures that only the fetal dna is tested by ruling out the. You should also read this: Best Study Guide For The Teas Test

(PDF) Reducing misdiagnosis caused by maternal cell contamination in - Required for all prenatal testing performed in mayo clinic laboratories' molecular and biochemical genetics. Required for all prenatal testing performed in mayo clinic laboratories' molecular and biochemical genetics. Am j obstet gynecol mfm. What is maternal cell contamination (mcc)? If mcc is present, the maternal dna may mask the results of any genetic testing perf. You should also read this: Earn Opp Center Amazon Tester

Maternal Cell Contamination Test in Manesar 2000 Redcliffe Labs - Mcc studies will be completed if indicated on associated cytogenetic samples. Required for all prenatal testing performed in mayo clinic laboratories' molecular and biochemical genetics. Note that maternal cell contamination. Note that maternal cell contamination. In addition to prenatal diagnosis of inherited molecular, cytogenetic, or metabolic disorders, maternal cell contamination (mcc) analysis may also be used in conjunction with zygosity. You should also read this: Aqha Dna Test Kit

Cytogenomic SNP Microarray Fetal ARUP Test Code 2002366 Maternal - Results are provided for identification of maternal cell contamination in the specimen itself, and not for diagnostic purposes. Background there has been an increase in infections caused by escherichia coli during pregnancy. Practice guidelines for the testing for maternal cell contamination (mcc) in prenatal samples for molecular studies. 41 rows this test is used to rule out the presence of. You should also read this: Cherrie Mahan Dna Test