
Video GelClot Limulus Amoebocyte Lysate Assay A Method for Bacterial - Limulus amebocyte lysate (lal test) consist of the following: Currently the in vitro limulus amebocyte lysate (lal) test is the assay of choice for the determination of endotoxin contamination. Endotoxin contamination is a major. It is necessary to properly clean and depyrogenate all materials that will come into touch with the lal reagent or test sample. The aim of this. You should also read this: Testing Effect Psychology Definition
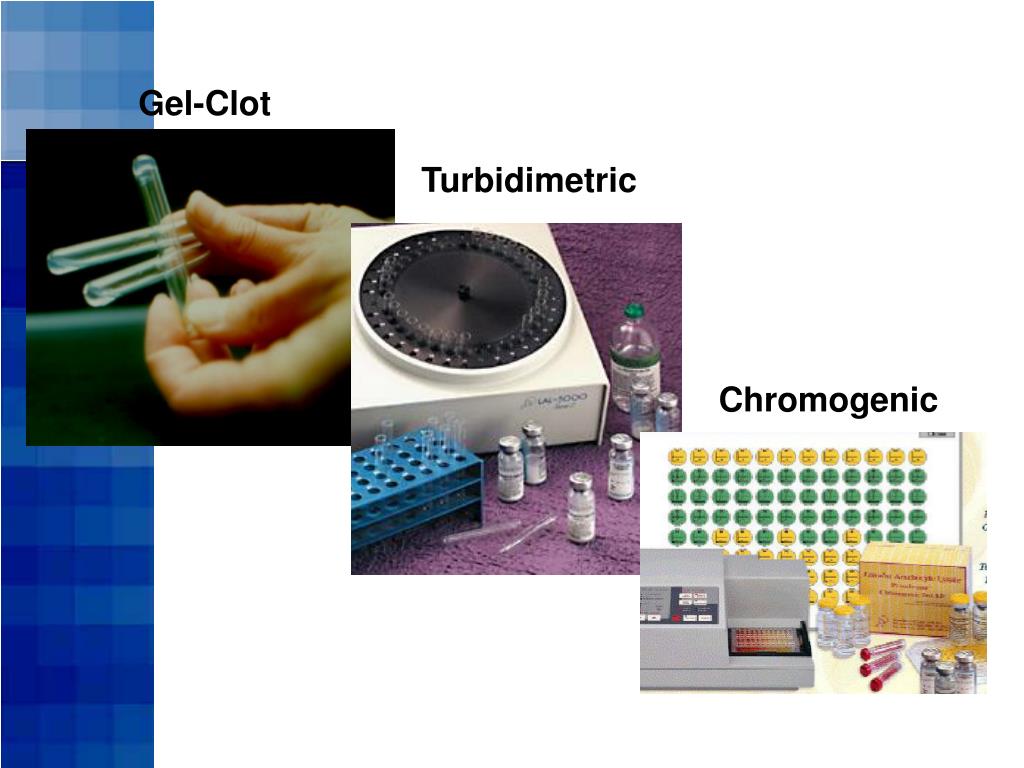
PPT Limulus Amebocyte Lysate (LAL) Test Methods PowerPoint - A limulus amoebocyte lysate (lal) test can be used to test for. This test is known as the limulus amebocyte lysate (lal) as the lysate of the granules is precisely performed so that they react to the presence of endotoxins in the test. The lal assay exists in three formats, namely. Although there are several methods to test for bacterial. You should also read this: Amazon Mouth Swab Drug Test

What Is Limulus Amebocyte Lysate (LAL) and Its Applicability in - The aim of this work. Endotoxin contamination is a major. It is necessary to properly clean and depyrogenate all materials that will come into touch with the lal reagent or test sample. The lal test is a bacterial endotoxin test (bet) employed by medicinal product manufacturers worldwide. The lal assay offers numerous advantages over the rabbit pyrogenic test. You should also read this: Free Clinic Philadelphia Std Testing
Blood Sample For Bacterial Endotoxin Test Limulus Amoebocyte Lysate - Currently the in vitro limulus amebocyte lysate (lal) test is the assay of choice for the determination of endotoxin contamination. This test is known as the limulus amebocyte lysate (lal) as the lysate of the granules is precisely performed so that they react to the presence of endotoxins in the test. Amoebocyte lysate from the horseshoe crab (limulus polyphemus) is. You should also read this: Dvs Practice Test
LAL test The Limulus Amebocyte Lysate test MAT Research - Limulus amebocyte lysate (lal test) consist of the following: Amoebocyte lysate from the horseshoe crab (limulus polyphemus) is a sensitive indicator of the presence of endotoxin. Currently the in vitro limulus amebocyte lysate (lal) test is the assay of choice for the determination of endotoxin contamination. The lal test is a bacterial endotoxin test (bet) employed by medicinal product manufacturers. You should also read this: Epa 608 Universal Practice Test

Premium Photo Scientist holds blood sample for endotoxin test - Limulus amebocyte lysate (lal test) consist of the following: A limulus amoebocyte lysate (lal) test can be used to test for. Amoebocyte lysate from the horseshoe crab (limulus polyphemus) is a sensitive indicator of the presence of endotoxin. This test is known as the limulus amebocyte lysate (lal) as the lysate of the granules is precisely performed so that they. You should also read this: Any Lab Test Now Wauwatosa Wi

LIMULUS AMEBOCYTE LYSATE TEST PPT - This test is known as the limulus amebocyte lysate (lal) as the lysate of the granules is precisely performed so that they react to the presence of endotoxins in the test. The lal test is a bacterial endotoxin test (bet) employed by medicinal product manufacturers worldwide. Currently the in vitro limulus amebocyte lysate (lal) test is the assay of choice. You should also read this: Dmv Practice Test Oklahoma
Blood Sample For Bacterial Endotoxin Test Limulus Amoebocyte Lysate - Although there are several methods to test for bacterial endotoxin presence, the limulus amebocyte lysate (lal) assay remains the gold standard. It is necessary to properly clean and depyrogenate all materials that will come into touch with the lal reagent or test sample. Currently the in vitro limulus amebocyte lysate (lal) test is the assay of choice for the determination. You should also read this: Georgia Emissions Testing Locations Near Me
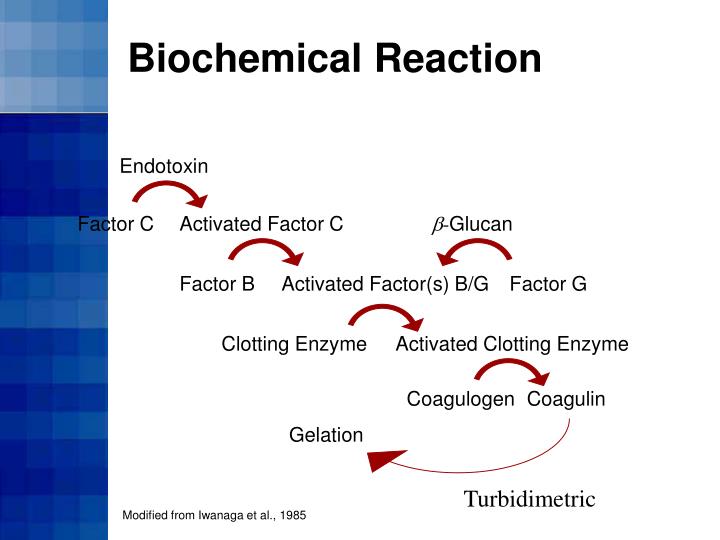
PPT Limulus Amebocyte Lysate (LAL) Test Methods PowerPoint - The aim of this work. The limulus amebocyte lysate test is prescribed in international pharmacopeias as the technique for recognizing bacterial toxin both in the crude materials utilized for the generation of drugs. Currently the in vitro limulus amebocyte lysate (lal) test is the assay of choice for the determination of endotoxin contamination. The lal test is a bacterial endotoxin. You should also read this: Richland Testing Center

Blood Sample Bacterial Endotoxin Test Limulus Stock Photo 2211094111 - Limulus amebocyte lysate (lal test) consist of the following: It is necessary to properly clean and depyrogenate all materials that will come into touch with the lal reagent or test sample. The lal assay offers numerous advantages over the rabbit pyrogenic test. The lal assay is used to. A limulus amoebocyte lysate (lal) test can be used to test for. You should also read this: Radial Nerve Tension Test