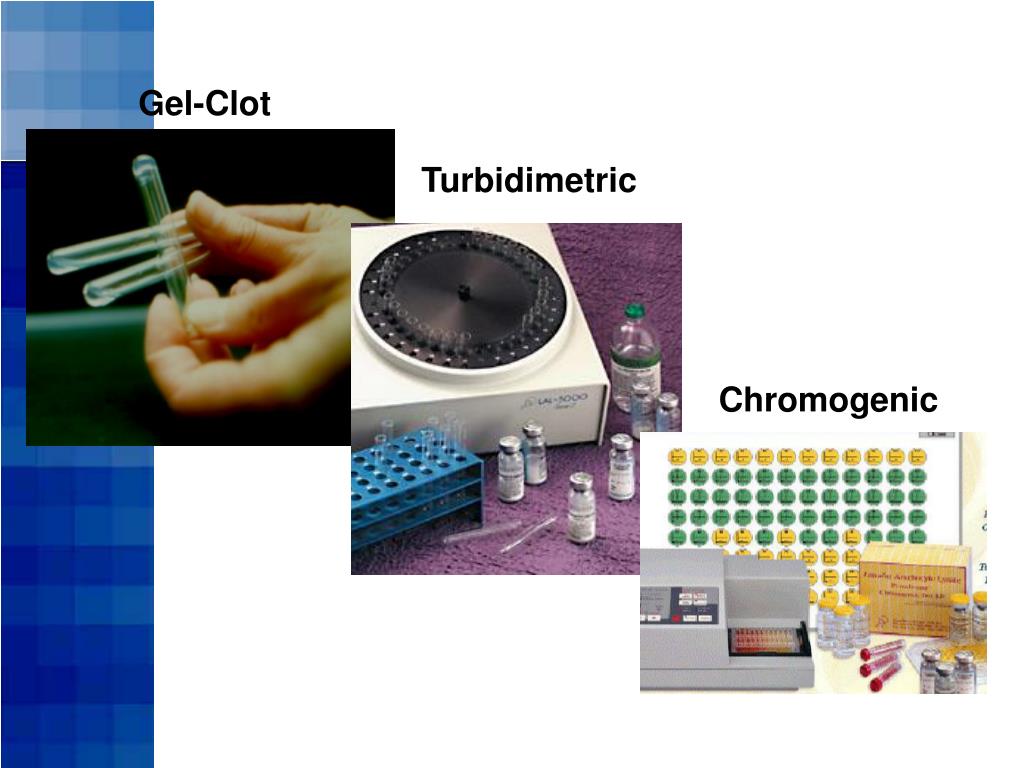
PPT Limulus Amebocyte Lysate (LAL) Test Methods PowerPoint - The lal assay exists in three formats, namely. It allows for in vitro endotoxin testing, which has. Amoebocyte lysate from the horseshoe crab (limulus polyphemus) is a sensitive indicator of the presence of endotoxin. Specifically, the lal test is a means of detecting and in some cases quantifying the. Explore key insights into limulus amebocyte lysate, its role in endotoxin. You should also read this: Cypress/skip-test

What Is Limulus Amebocyte Lysate (LAL) and Its Applicability in - The bacterial endotoxin detection method using limulus amebocyte lysate (lal). The limulus amebocyte lysate test is prescribed in international pharmacopeias as the technique for recognizing bacterial toxin both in the crude materials utilized for the generation of drugs. Limulus amebocyte lysate test using chromogenic methods of endotoxin detection. The lal test is applied in the testing of pharmaceuticals, medical devices,. You should also read this: Can Celsius Make You Fail A Drug Test
Blood Sample For Bacterial Endotoxin Test Limulus Amoebocyte Lysate - The lal assay exists in three formats, namely. This test is known as the limulus amebocyte lysate (lal) as the lysate of the granules is precisely performed so that they react to the presence of endotoxins in the test. Lal reagent reacts with the. Specifically, the lal test is a means of detecting and in some cases quantifying the. A. You should also read this: Which Gas Stations Sell Pregnancy Tests

Premium Photo Scientist holds blood sample for endotoxin test - Lal reagent reacts with the. It allows for in vitro endotoxin testing, which has. The lal assay exists in three formats, namely. The limulus amebocyte lysate test is prescribed in international pharmacopeias as the technique for recognizing bacterial toxin both in the crude materials utilized for the generation of drugs. The lal test is a bacterial endotoxin test (bet) employed. You should also read this: Does Methocarbamol Show Up In A Urine Drug Test
PPT Limulus Amebocyte Lysate (LAL) Test Methods PowerPoint - Limulus amebocyte lysate test using chromogenic methods of endotoxin detection. Specifically, the lal test is a means of detecting and in some cases quantifying the. Amoebocyte lysate from the horseshoe crab (limulus polyphemus) is a sensitive indicator of the presence of endotoxin. Limulus amebocyte lysate test is an aqueous extract of blood cells (amoebocytes) which obtain from the horseshoe crab. You should also read this: Mind Betterme World Trauma Test Test Español

Figure 4 from INNOVATIVE MECHANISM OF LIMULUS AMEBOCYTE LYSATE - Explore key insights into limulus amebocyte lysate, its role in endotoxin testing, influencing factors, and applications in laboratory settings. Amoebocyte lysate from the horseshoe crab (limulus polyphemus) is a sensitive indicator of the presence of endotoxin. Limulus amebocyte lysate test using chromogenic methods of endotoxin detection. Almost since its discovery, limulus amoebocyte lysate (lal) testing has been an important part. You should also read this: Dream Meaning Of Positive Pregnancy Test

LIMULUS AMEBOCYTE LYSATE TEST PPT - This test is known as the limulus amebocyte lysate (lal) as the lysate of the granules is precisely performed so that they react to the presence of endotoxins in the test. Limulus amebocyte lysate test using chromogenic methods of endotoxin detection. Currently the in vitro limulus amebocyte lysate (lal) test is the assay of choice for the determination of endotoxin. You should also read this: Math Test 9 90 8 72 Answer
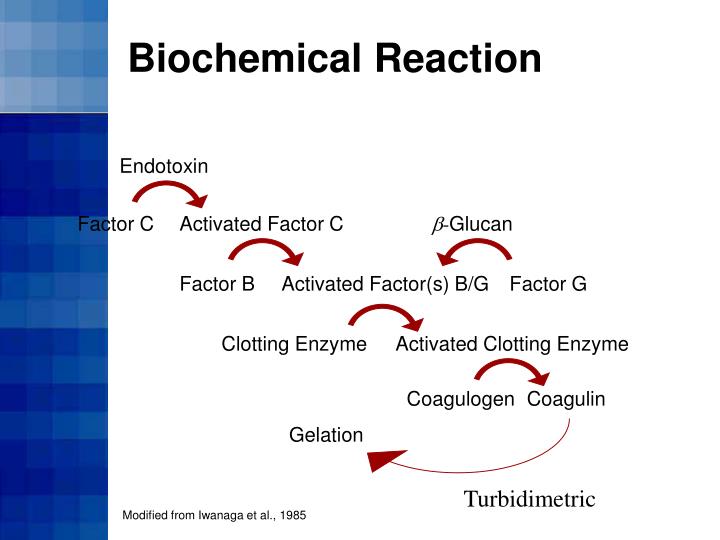
PPT Limulus Amebocyte Lysate (LAL) Test Methods PowerPoint - It allows for in vitro endotoxin testing, which has. The lal test is a bacterial endotoxin test (bet) employed by medicinal product manufacturers worldwide. The bacterial endotoxin detection method using limulus amebocyte lysate (lal). Almost since its discovery, limulus amoebocyte lysate (lal) testing has been an important part of the pharmaceutical quality control toolkit. The lal assay exists in three. You should also read this: Gallbladder Nuclear Test
LAL test The Limulus Amebocyte Lysate test MAT Research - A limulus amoebocyte lysate (lal) test can be used to test for. Almost since its discovery, limulus amoebocyte lysate (lal) testing has been an important part of the pharmaceutical quality control toolkit. Limulus amebocyte lysate test using chromogenic methods of endotoxin detection. It allows for in vitro endotoxin testing, which has. Lal reagent reacts with the. You should also read this: Parallel Parking Test Texas
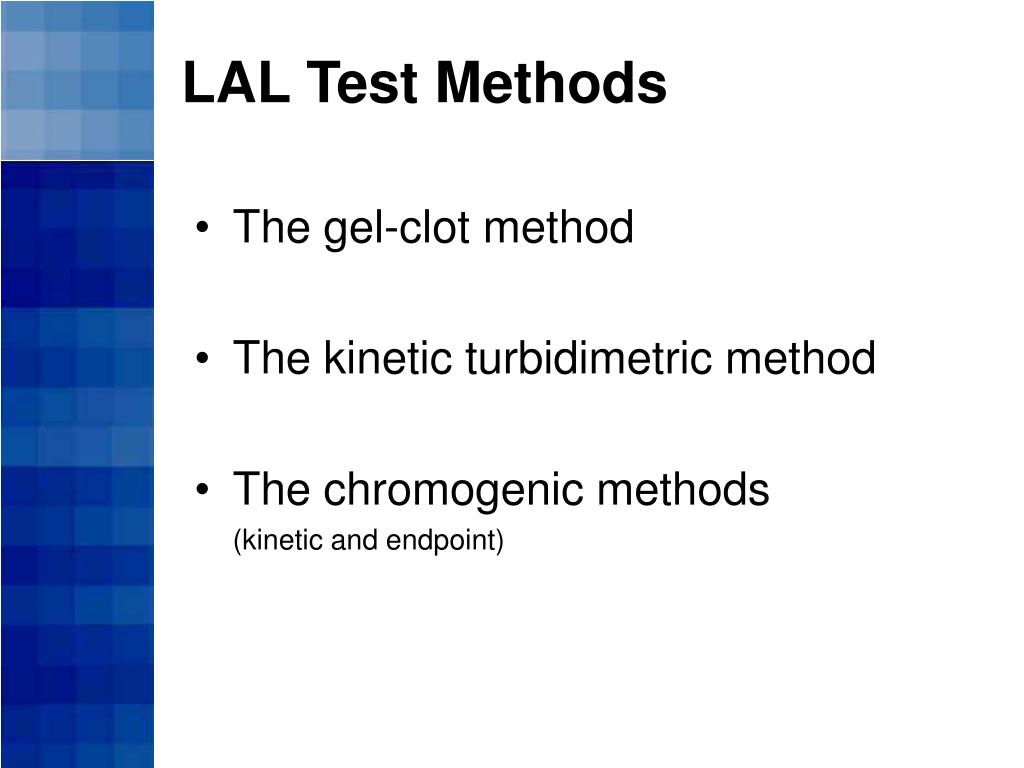
PPT Limulus Amebocyte Lysate (LAL) Test Methods PowerPoint - The limulus amebocyte lysate test is prescribed in international pharmacopeias as the technique for recognizing bacterial toxin both in the crude materials utilized for the generation of drugs. It allows for in vitro endotoxin testing, which has. Limulus amebocyte lysate test using chromogenic methods of endotoxin detection. A limulus amoebocyte lysate (lal) test can be used to test for. The. You should also read this: Bltps Damage Testing