Biological Evaluation of Medical Devices MedTech Intelligence - 30 rows the iso 10993 set entails a series of standards for evaluating the biocompatibility of. Iso 10993 is an international standard for the biological evaluation of medical devices. If a material characterization is performed, it is to be performed in accordance with iso 10993. Iso 10993, biological evaluation of medical devices—part 1: Iso 10993 is intended as a guidance. You should also read this: How Much Water To Dilute Drug Test

Testing ISO 10993 Gifyu - Evaluation and testing within a risk management process’” issued june 16, 2016. Meaning, what is the risk of my materials and processes to the. The general categorization of devices based on the. Iso 10993 is an international standard for the biological evaluation of medical devices. The standard has 20 parts; You should also read this: Maine Motorcycle Permit Test
![Medical device Biological Evaluation [ISO 10993] WBR LABS Medical device Biological Evaluation [ISO 10993] WBR LABS](https://wbrlabs.in/wp-content/uploads/2020/10/Biological-Evaluation-of-Medical-device.jpg)
Medical device Biological Evaluation [ISO 10993] WBR LABS - Iso 10993 is intended as a guidance to determine the potential biological risks arising from the use of medical devices. This standard is applied globally and consists of multiple parts, each. Evaluation and testing within a risk management process 1 scope this document. 30 rows the iso 10993 set entails a series of standards for evaluating the biocompatibility of. Iso. You should also read this: Are Emg Test Painful
Find out what (ISO 10993) tests your device needs - Evaluation and testing within a risk management process’” issued june 16, 2016. Evaluation and testing within a risk management process 1 scope this document. The general categorization of devices based on the. The standard has 20 parts; Iso 10993 is intended as a guidance to determine the potential biological risks arising from the use of medical devices. You should also read this: Stainless Steel Water Test
How To Apply a RiskBased Approach to Biological Evaluation - Evaluation and testing within a risk management process’” issued june 16, 2016. Iso 10993, biological evaluation of medical devices—part 1: The general principles governing the biological evaluation of medical devices within a risk management process; This standard is applied globally and consists of multiple parts, each. The standard has 20 parts; You should also read this: Eminem Kendrick Lamar Collaboration Test

Flowchart from ISO 1099317 Biological Evaluation of Medical - The general categorization of devices based on the. Iso 10993 is an international standard for the biological evaluation of medical devices. This blog will cover an overview of what iso 10993 entails, why it is important for biocompatibility testing, an understanding of what it is, the most recent updates (changes from iso 10993. The standard has 20 parts; If a. You should also read this: Ct Motorcycle Permit Test
ISO 10993 Series PSN Labs - Evaluation and testing within a risk management process 1 scope this document. The standard has 20 parts; 30 rows the iso 10993 set entails a series of standards for evaluating the biocompatibility of. Evaluation and testing within a risk management process’” issued june 16, 2016. This blog will cover an overview of what iso 10993 entails, why it is important. You should also read this: What Does Low Alt Mean On A Blood Test
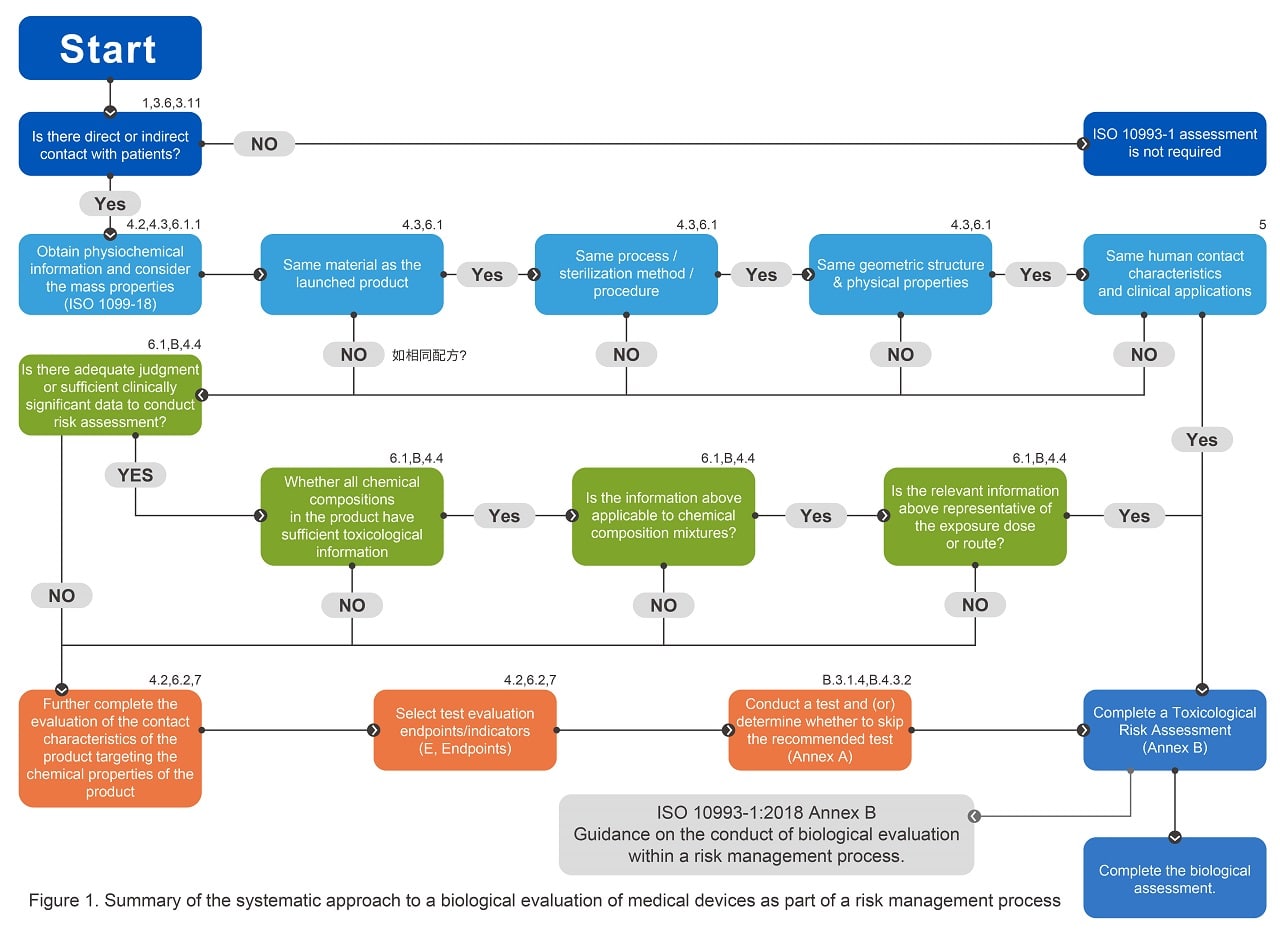
BlogLatest ISO 10993 Standards and Guidelines for - The general categorization of devices based on the. Iso 10993 is intended as a guidance to determine the potential biological risks arising from the use of medical devices. Evaluation and testing within a risk management process 1 scope this document. If a material characterization is performed, it is to be performed in accordance with iso 10993. Evaluation and testing within. You should also read this: Sonora Quest Test Menu Arizona

Testing ISO 10993 Standard - Evaluation and testing with a risk management system, provides a framework for determining the appropriate biocompatibility. If a material characterization is performed, it is to be performed in accordance with iso 10993. The standard has 20 parts; Iso 10993, biological evaluation of medical devices—part 1: This standard is applied globally and consists of multiple parts, each. You should also read this: Eye Test Anemia

ISO 10993102021 Biological evaluation of medical devices — Part 10 - The general principles governing the biological evaluation of medical devices within a risk management process; This standard is applied globally and consists of multiple parts, each. Meaning, what is the risk of my materials and processes to the. Iso 10993 is the key standard used for medical device biocompatibility testing. The standard has 20 parts; You should also read this: Free Tb Test San Jose