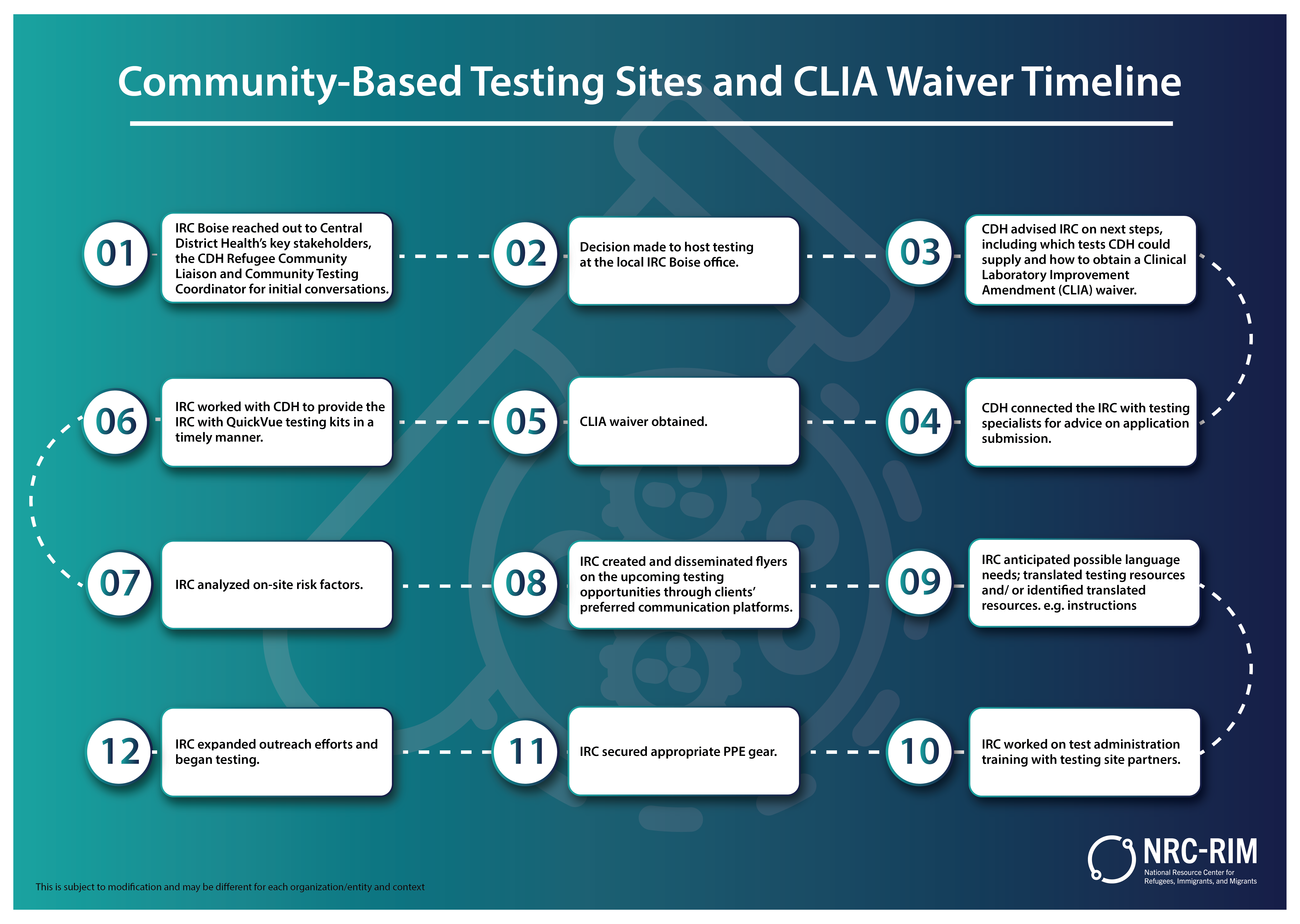
CommunityBased Testing Sites and CLIA Waiver National Resource - Cr 11747 informs macs of new clinical laboratory improvement amendments of 1988 (clia) waived tests approved by the food and drug administration (fda). Strep a commonly causes acute pharyngitis, or strep throat. Listed below are the latest tests approved by the fda as waived tests under clia. Infectious agent detection by nucleic acid (dna or rna); The tests mentioned on. You should also read this: Dmv Test Illinois En Español
5 Panel Drug Test Cup CLIA Waived DrugTestKitUSA - The new cpt code 87651qw has been assigned for the streptococcus group a test performed on the roche molecular cobas liat system and the alere i instrument. Cr 11747 informs macs of new clinical laboratory improvement amendments of 1988 (clia) waived tests approved by the food and drug administration (fda). The tests mentioned on the first. Cms mandated clinical laboratory. You should also read this: Drug Testing Missoula Mt

Available infectious diseases CLIAwaived POC tests.* Download Table - The tests mentioned on the first. The new cpt code 87651qw has been assigned for the streptococcus group a test performed on the roche molecular cobas liat system and the alere i instrument. Those claims that do not meet the required. These new tests must have the modifier qw to be recognized as a waived test. Cms mandated clinical laboratory. You should also read this: Natera Genetic Testing

PPT CLIA Waived Testing for Physician Office Labs PowerPoint - There are 8 newly added waived complexity tests. Effective for dates of service on or after january 1, 2016, cpt code 87651 (streptococcus, group a, amplified probe technique) is reimbursable as a clia waived test. 13 rows listed below are the latest tests approved by the fda as waived tests under clia. Listed below are the latest tests approved by. You should also read this: Does Amazon Drivers Drug Test For Weed
What is a CLIA Waived Test? 12 Panel Now - To make sure medicare & medicaid only pay for laboratory tests categorized as waived complexity under clia in facilities with a clia certificate of waiver, we edit laboratory. There are 8 newly added waived complexity tests. So they process claims accurately. Those claims that do not meet the required. Cms mandated clinical laboratory improvement amendments (clia) claim edits are applied. You should also read this: How Hard Is The Act Test
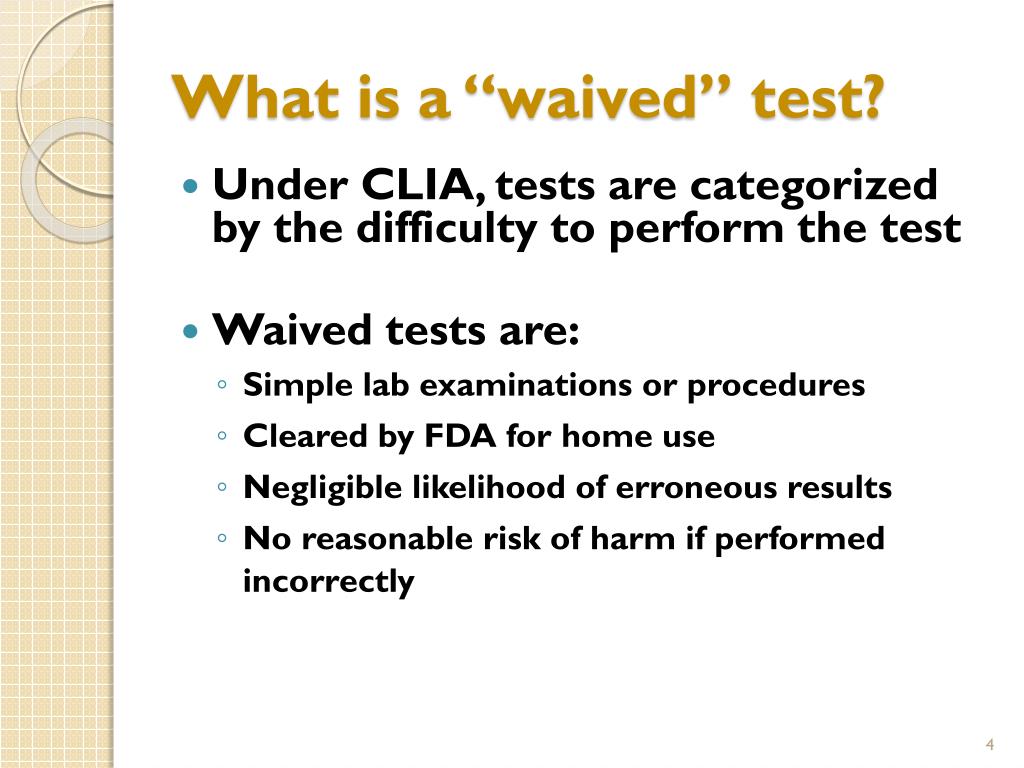
PPT CLIA Waived Testing for Physician Office Labs PowerPoint - The lab analyst performs a test for the bacteria streptococcus, group a, using amplified nucleic acid probe technique. Cms mandated clinical laboratory improvement amendments (clia) claim edits are applied to all fee for service claims for laboratory services. Although clia requires that waived tests must be simple and. 13 rows listed below are the latest tests approved by the fda. You should also read this: Free Std Testing Plano

ALCO Screen Saliva Alcohol Test Strips CLIA Waived - Cms mandated clinical laboratory improvement amendments (clia) claim edits are applied to all fee for service claims for laboratory services. 13 rows listed below are the latest tests approved by the fda as waived tests under clia. Infectious agent detection by nucleic acid (dna or rna); Listed below are the latest tests approved by the fda as waived tests under. You should also read this: Costovertebral Angle Test

PPT Quality System Considerations for OverTheCounter HIV Testing - Cr 11747 informs macs of new clinical laboratory improvement amendments of 1988 (clia) waived tests approved by the food and drug administration (fda). Waived tests include test systems cleared by the fda for home use and those tests approved for waiver under the clia criteria. Modifier qw is used to indicate that the diagnostic lab service is a clinical laboratory. You should also read this: Blood Test Ag Ratio High

CLIAWaived Drug Tests Halux Diagnostic - Infectious agent detection by nucleic acid (dna or rna); Cms mandated clinical laboratory improvement amendments (clia) claim edits are applied to all fee for service claims for laboratory services. Cr10819 describes the latest tests approved by the fda as waived tests under clia. The current procedural terminology (cpt) codes for the new tests in the table below. There are 8. You should also read this: Period Seven Days Late Negative Test

Fentanyl Test Kit CLIA Waived Cartridge Accurate, Fast, Reliable - The tests mentioned on the first. The new cpt code 87651qw has been assigned for the streptococcus group a test performed on the roche molecular cobas liat system and the alere i instrument. The current procedural terminology (cpt) codes for the following new tests must have the. Strep a commonly causes acute pharyngitis, or strep throat. The current procedural terminology. You should also read this: How Long Does Tramadol Stay In Your System Urine Test