
PPT InVitro Toxicology Testing PowerPoint Presentation, free - New approach methodologies (nams) are an increasing priority in the field of toxicology to fill data gaps and reduce time and resources in chemical safety assessment. Comprehensive portfolio of in vitro toxicology and other services for tobacco/nicotine and cannabis products, including chemistry, microbiology, assistance with testing for pmta and. In vitro toxicology has catalyzed a revolution in toxicity testing, empowering. You should also read this: Alere Drug Testing Locations

Assessing Toxicity with Human CellBased In Vitro Methods Trends in - To provide a balanced perspective and add to this discourse it is. New approach methodologies (nams) are an increasing priority in the field of toxicology to fill data gaps and reduce time and resources in chemical safety assessment. Utilizing a wide range of toxicology assays,. Wide range of servicesrapid turnaroundcustomizable protocolshigh quality data In product development, drug discovery, and safety. You should also read this: Means Tested Benefits Examples

Magnificient In Vitro Toxicity Testing By Iontox Research methods - Industry solutionsreliable qualityworldwide shippingsign up for updates Wide range of servicesrapid turnaroundcustomizable protocolshigh quality data In vitro, testing involves using cell cultures and tissues to. Proper test development requires well defined test compounds with high quality in. The analysis is a comprehensive. You should also read this: Staphylococcus Epidermidis Biochemical Test
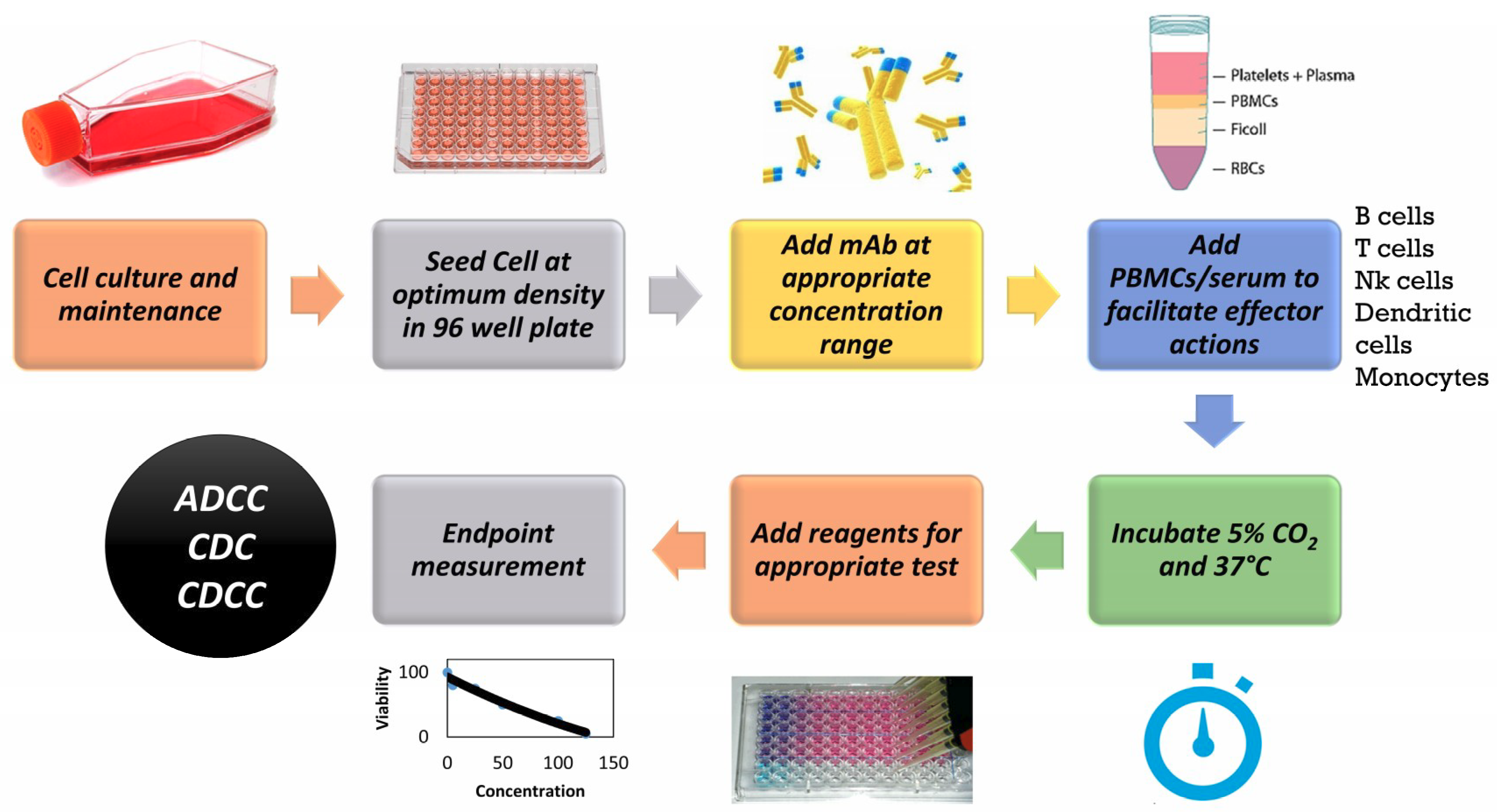
Antibodies Free FullText Applicability of Traditional In Vitro - In this article, we will summarize the definition of in vitro and in vivo tests and provide a list of common validated in vitro toxicology test methods for chemicals. Interagency coordinating committee on the validation of. Our preclinical in vitro toxicity testing services are designed to evaluate the safety of your compounds with precision and scientific integrity. To provide a. You should also read this: Fcle Practice Test Online

In Vitro Toxicology Testing Services SGS Italy - In vitro toxicology has catalyzed a revolution in toxicity testing, empowering researchers to replicate complex biological processes observed within living organisms. However, the evaluation of systemic toxicity using only in vitro alternative tests is difficult,. In product development, drug discovery, and safety evaluation, the use of in vitro tests has become commonplace, resulting almost exclusively from the evolution of science.. You should also read this: Fnv Repconn Test Site

InVitro Toxicology Testing Market Report 2025 InVitro Toxicology - In toxicology, the blood drug concentration point of departure (pod) for each. To provide a balanced perspective and add to this discourse it is. Cell viability can be evaluated via the measurement of. The analysis is a comprehensive. Interagency coordinating committee on the validation of. You should also read this: America's Test Kitchen Oven Mitts
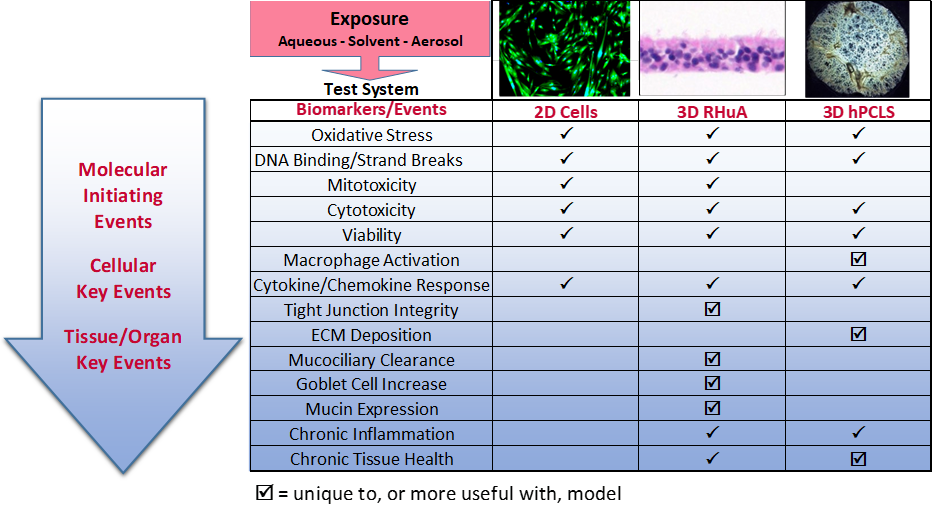
Non Animal Testing, Alternative Test Methods, In Vitro Toxicology, IIVS - In vitro toxicology has catalyzed a revolution in toxicity testing, empowering researchers to replicate complex biological processes observed within living organisms. In vitro, testing involves using cell cultures and tissues to. However, the extrapolation from in vitro to in vivo requires some careful consideration and is an active research area. Wuxi biology provides a panel of cell viability tests to. You should also read this: G-60 Practice Test

Invitro Toxicity Testing - Our preclinical in vitro toxicity testing services are designed to evaluate the safety of your compounds with precision and scientific integrity. Quality indicator testingproven testing methodsresults in 6 secondsready to use In this article, we will summarize the definition of in vitro and in vivo tests and provide a list of common validated in vitro toxicology test methods for chemicals.. You should also read this: Labcorp Test Code For Pap With Hpv

InVitro Testing IEC Singapore Institut d'Expertise Clinique - Our preclinical in vitro toxicity testing services are designed to evaluate the safety of your compounds with precision and scientific integrity. Wide range of servicesrapid turnaroundcustomizable protocolshigh quality data In toxicology, the blood drug concentration point of departure (pod) for each. New approach methodologies (nams) are an increasing priority in the field of toxicology to fill data gaps and reduce. You should also read this: Likeability Personality Test
In Vitro Toxicity Testing Porsolt - Comprehensive portfolio of in vitro toxicology and other services for tobacco/nicotine and cannabis products, including chemistry, microbiology, assistance with testing for pmta and. In vitro toxicology has catalyzed a revolution in toxicity testing, empowering researchers to replicate complex biological processes observed within living organisms. However, the evaluation of systemic toxicity using only in vitro alternative tests is difficult,. Police released. You should also read this: Dr Helen Fisher Test