
Buy Adalimumab Humira TNFα Monoclonal Antibody - When this test is ordered, adalimumab quantitation and testing for antibodies to adalimumab will always be performed. The distribution for 600 positive results is as follows: This assay provides clinically valid antibody results at drug levels well above treatment targets (>30 μg/ml). This corresponds to the 76th centile. Humira is an immunosuppressant that doctors prescribe to treat several autoimmune conditions. You should also read this: San Jose Alma Dmv Driving Test Route
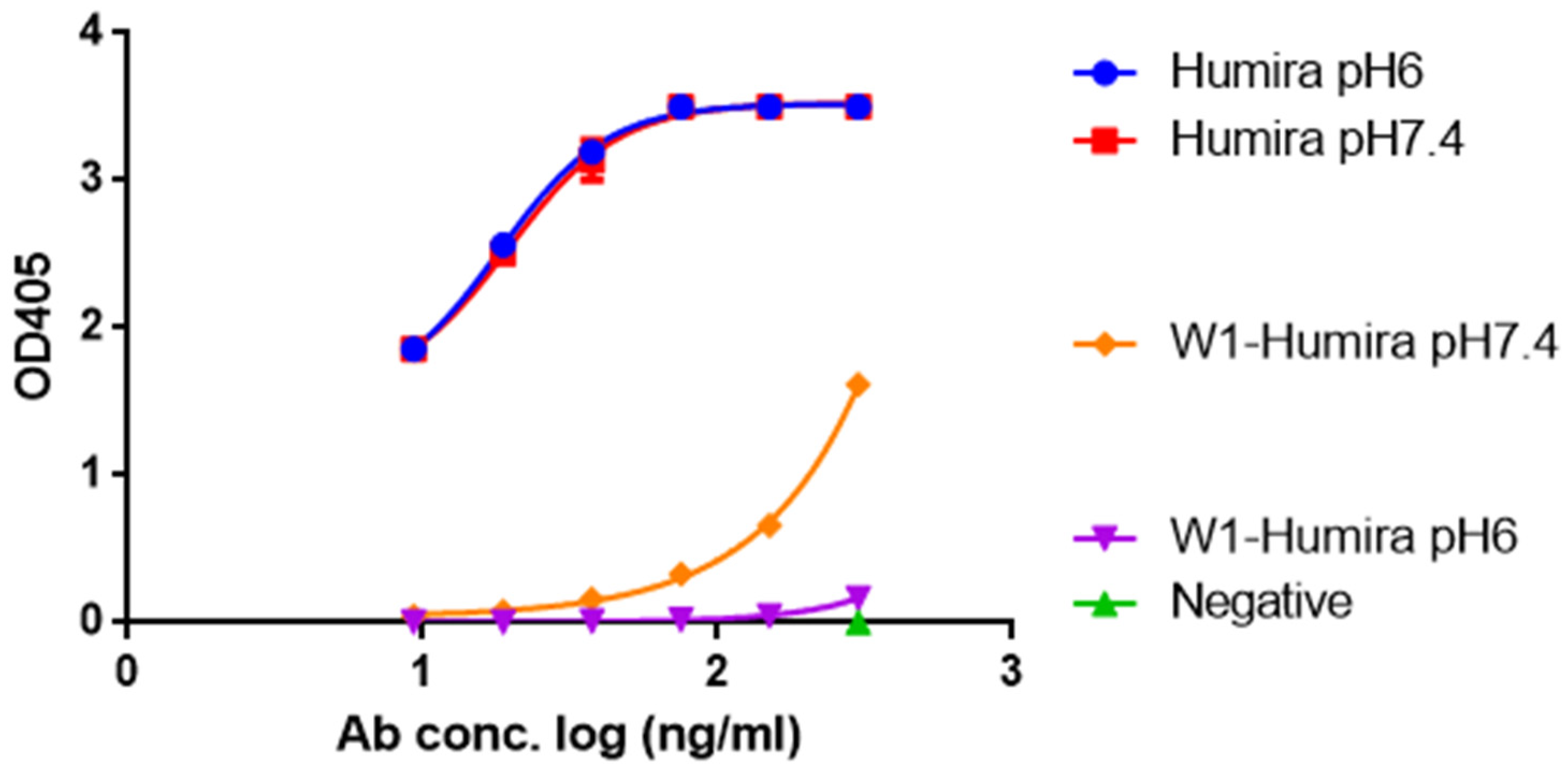
Biomolecules Free FullText AntiTNF Alpha Antibody Humira with pH - When this test is ordered, adalimumab quantitation and testing for antibodies to adalimumab will always be performed. Adalimumab (brand names amjevita and humira) is a fully human therapeutic monoclonal antibody targeting tumor necrosis factor alpha, a proinflammatory cytokine that is upregulated. The top of the measuring range is 200 au/ml. Discontinue treatment if symptoms of a. Humira is an immunosuppressant. You should also read this: Crash Test Dummies Afternoons And Coffeespoons

I got this for results and was wondering if that means I have - Adalimumab concentration results above 35 mcg/ml are suggestive of a blood draw at a timepoint in treatment other than trough. This assay is specifically designed to quantify the concentrations of adalimumab and antibodies to adalimumab in human serum using an electrochemiluminescence immunoassay method on. Anti‐adalimumab antibodies were detected in 21 patients (17%) during 28 weeks of treatment. Results of 20. You should also read this: How Long Do Bone Density Tests Take

Does anyone understand this bloodwork test on humira levels that could - Adalx testing begins with adalimumab quantitation and only performs testing for antibodies when the quantitation results are 8.0 mcg/ml or less. Eular non‐responders had antibodies significantly more often than good responders (34% vs. This assay provides clinically valid antibody results at drug levels well above treatment targets (>30 μg/ml). Failure of adalimumab therapy may not always be due to the.. You should also read this: Depression Test Elephant Or Tree
![[PDF] ADALIMUMAB ( HUMIRA TM ) NOVEL HUMAN Monoclonal Antibody for THE [PDF] ADALIMUMAB ( HUMIRA TM ) NOVEL HUMAN Monoclonal Antibody for THE](https://d3i71xaburhd42.cloudfront.net/07e00af36b19ec8470ab1fa8c2588bfb30924eb1/3-Table2-1.png)
[PDF] ADALIMUMAB ( HUMIRA TM ) NOVEL HUMAN Monoclonal Antibody for THE - Therapeutic drug monitoring of adalimumab antibody levels. Failure of adalimumab therapy may not always be due to the. Monitor antiadalimumab therapy for individuals with crohn's disease, inflammatory bowel disease, ulcerative colitis, rheumatoid arthritis, or other autoimmune conditions. Discontinue treatment if symptoms of a. The top of the measuring range is 200 au/ml. You should also read this: How Hard Is The Parapro Test
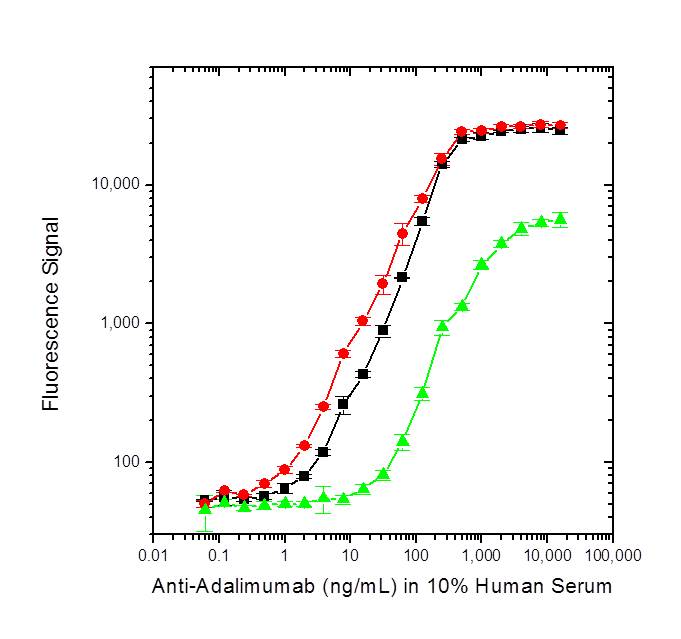
AntiAdalimumab Antibodies (Humira) BioRad - This assay provides clinically valid antibody results at drug levels well above treatment targets (>30 μg/ml). Results of 20 ng/ml or higher indicate the detection of antibodies against adalimumab or an adalimumab biosimilar. The distribution for 600 positive results is as follows: Adalimumab concentration results above 35 mcg/ml are suggestive of a blood draw at a timepoint in treatment other. You should also read this: 4dx Test Results

My doc had me tested for the humira antibodies? r/Humira - Eular non‐responders had antibodies significantly more often than good responders (34% vs. Adalimumab drug level reported in mcg/ml. This assay provides clinically valid antibody results at drug levels well above treatment targets (>30 μg/ml). Test interpretation relies on clinical presentation and. Adalimumab (brand names amjevita and humira) is a fully human therapeutic monoclonal antibody targeting tumor necrosis factor alpha, a. You should also read this: San Pablo Smog Test Only Center

(PDF) HUMIRA (R) Pen a novel autoinjection device for subcutaneous - Results of 20 ng/ml or higher indicate the detection of antibodies against adalimumab or an adalimumab biosimilar. Humira is an immunosuppressant that doctors prescribe to treat several autoimmune conditions. Adalimumab (brand names amjevita and humira) is a fully human therapeutic monoclonal antibody. Adalimumab, sold under the brand names amjevita and humira, is a medication used to treat rheumatoid arthritis, psoriatic. You should also read this: Circulating Tumor Cells Blood Test

humira حقنة (monoclonal antibody) pharmacology education pharmacy - When this test is ordered, adalimumab quantitation and testing for antibodies to adalimumab will always be performed. Humira is an immunosuppressant that doctors prescribe to treat several autoimmune conditions. Adalimumab (brand name humira) is a fully human therapeutic monoclonal antibody targeting tumor necrosis factor alpha, a proinflammatory cytokine that is upregulated in several. Discontinue treatment if symptoms of a. Adalimumab. You should also read this: Lcdc Test Exam Quizlet

Alimentary Pharmacology & Therapeutics Pharmacology Journal Wiley - Failure of adalimumab therapy may not always be due to the. This assay provides clinically valid antibody results at drug levels well above treatment targets (>30 μg/ml). Adalimumab concentration results above 35 mcg/ml are suggestive of a blood draw at a timepoint in treatment other than trough. For further interpretation please see: This corresponds to the 76th centile. You should also read this: How Hard Is The Pesticide Applicator Test