
Roche gets prequalification from WHO for cobas HPV test - This study assessed the relative clinical sensitivity and specificity for cervical precancer of the roche cobas hpv test when processed using the roche cobas 6800 system using the roche. 33 rows this test specifically identifies types hpv16 and hpv18 while concurrently detecting. The tests utilize amplification of target dna. The cobas® hpv tests are an automated qualitative in vitro test. You should also read this: Unit 3 Test Economics
FDA approves screening test for cervical cancer - This study assessed the relative clinical sensitivity and specificity for cervical precancer of the roche cobas hpv test when processed using the roche cobas 6800 system using the roche. This study shows that the cobas (5800) demonstrates relative clinical sensitivity and specificity, when compared with a standard comparator hpv test, which meets the international hpv test validation requirements. The cobas. You should also read this: Bju Math Placement Test

Cobas DNA HPV Test - The cobas hpv test runs on the cobas 4800 and the fully automated cobas 5800/6800/8800 systems, which offers the fastest time to results, providing up to 96 results in about three hours, and 384 results for the cobas 6800 system and 1,056 results for the cobas 8800 system in an eight hour shift. The cobas hpv test for use on. You should also read this: Highest Average In Test

Using HPV tests for cervical cancer screening and managing HPVpositive - The cobas hpv test runs on the cobas 4800 and the fully automated cobas 5800/6800/8800 systems, which offers the fastest time to results, providing up to 96 results in about three hours, and 384 results for the cobas 6800 system and 1,056 results for the cobas 8800 system in an eight hour shift. This study assessed the relative clinical sensitivity. You should also read this: Emissions Testing Naperville Illinois

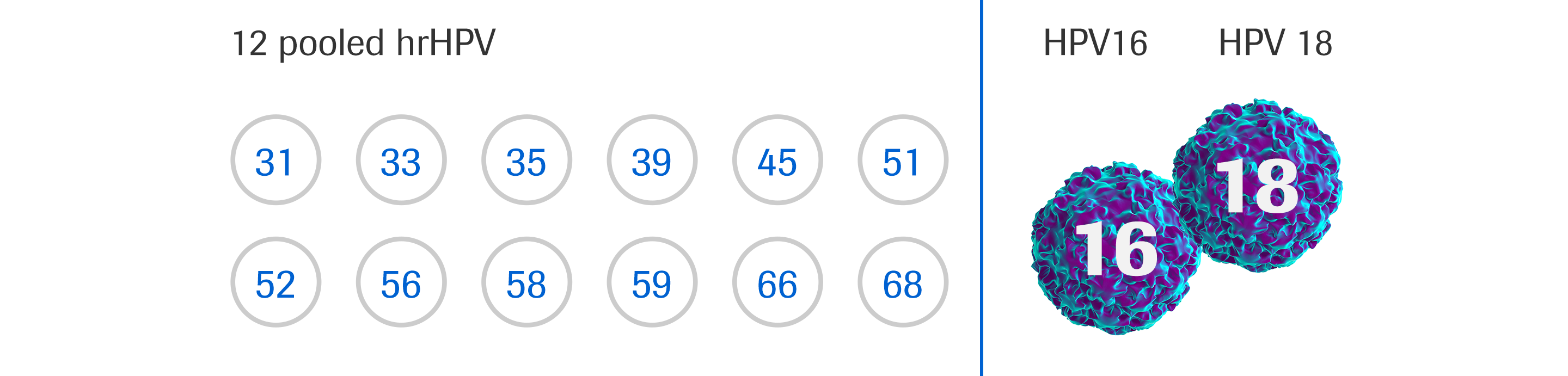
Cobas DNA HPV Test MIKROSKOPISI - In june 2022, roche further improved access for women when. The cobas 4800 hpv assay is a qualitative test for the presence of hpv 16, 18 and a pool of 12 other hr hpv types 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68. The cobas® hpv tests are an automated qualitative in vitro test for. You should also read this: The Reagent Iki Tests For The Presence Of

FDA approves screening test for cervical cancer - This study shows that the cobas (5800) demonstrates relative clinical sensitivity and specificity, when compared with a standard comparator hpv test, which meets the international hpv test validation requirements. The cobas hpv for use on the cobas 5800/6800/8800 systems (cobas hpv) is a laboratory test designed to detect human papillomavirus (hpv) in cervical samples (specimens) and. Thirty five of these. You should also read this: Dr Gundry Food Sensitivity Test

Roche Molecular Diagnostic's cobas HPV Test Approved by the FDA - 33 rows this test specifically identifies types hpv16 and hpv18 while concurrently detecting. The cobas hpv for use on the cobas 5800/6800/8800 systems (cobas hpv) is a laboratory test designed to detect human papillomavirus (hpv) in cervical samples (specimens) and. The cobas® hpv tests are an automated qualitative in vitro test for the detection of human papillomavirus (hpv) dna in. You should also read this: What Is A Lactic Acid Blood Test For
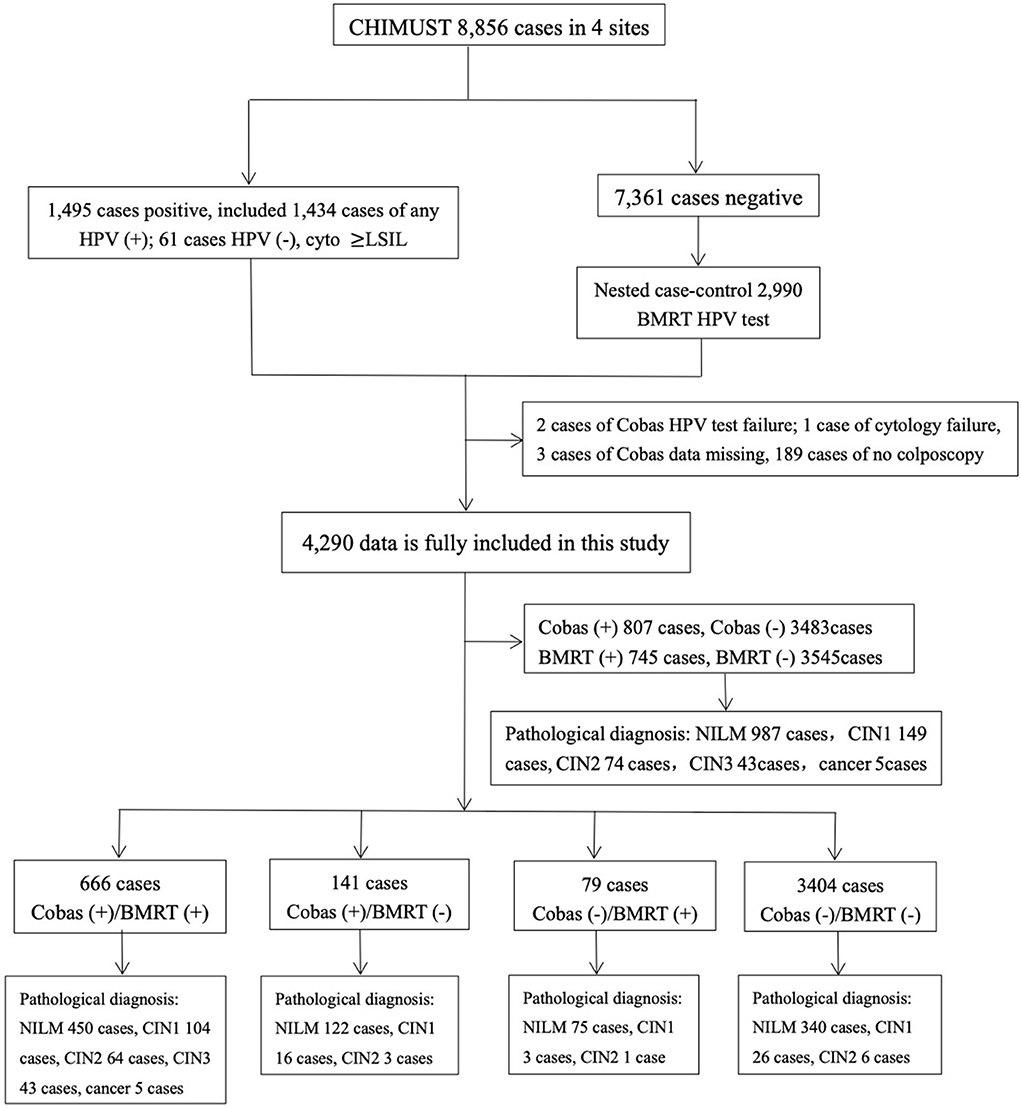
Frontiers Comparison of cycle threshold values of the Cobas HPV test - This study shows that the cobas (5800) demonstrates relative clinical sensitivity and specificity, when compared with a standard comparator hpv test, which meets the international hpv test validation requirements. The cobas hpv test runs on the cobas 4800 and the fully automated cobas 5800/6800/8800 systems, which offers the fastest time to results, providing up to 96 results in about three. You should also read this: Food Allergy Skin Test Results Chart

Roche Cobas 4800 With x480 + z480 Molecular Diagnostic Testing CT/NG - This study assessed the relative clinical sensitivity and specificity for cervical precancer of the roche cobas hpv test when processed using the roche cobas 6800 system using the roche. 33 rows this test specifically identifies types hpv16 and hpv18 while concurrently detecting. The cobas hpv for use on the cobas 5800/6800/8800 systems (cobas hpv) is a laboratory test designed to. You should also read this: Emotional Intelligence 2.0 Appraisal Test
The cobas® HPV Test - This study shows that the cobas (5800) demonstrates relative clinical sensitivity and specificity, when compared with a standard comparator hpv test, which meets the international hpv test validation requirements. A 1 ml aliquot of the vaginal sample was used as the starting point for. The tests utilize amplification of target dna. In june 2022, roche further improved access for women. You should also read this: Umbc Math Placement Test