
Set Of Flame Test Colors With A Bunsen Burner Illustrations Of Various - These photons have a frequency (light color) that is a characteristic of the element. Flame emission spectroscopy is an example of an instrumental method used to analyse metal ions in solutions. Perform the flame test under the direction or supervision of chemistry teachers. A sample is heated in a flame, and the light emitted from. It requires a sample volume. You should also read this: Wire Pull Test

EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:2.7.2 Flame Tests - Dropping to a more stable energy state involves the release of photons. When a sample is heated in a bunsen burner. This method is straightforward and requires. Flame photometers (often referred to as a flame emission spectroscope) is an instrumental method used to analyse metal ions. The flame test is a procedure used in chemistry to detect the presence of. You should also read this: Connecting Math Concepts Placement Test Level D

aluminum dust flame YouTube - Flame test is an analytical technique used to detect the presence of metal ions based on their emission spectrum produced when the sample is in contact with the flame. However, it is only good for group 1 compounds, and the colour. The aluminium oxide flame test is a quick and simple way to identify certain metal ions in a compound.. You should also read this: How Long Do Clear Blue Pregnancy Tests Take

chemistry properties flametest Flame test for metal ions YouTube - Dropping to a more stable energy state involves the release of photons. A flame test is a qualitative analytical procedure used to detect the presence of specific metal ions in compounds based on the characteristic colors they emit when heated in a flame. The aluminium oxide flame test is a quick and simple way to identify certain metal ions in. You should also read this: Mann Whitney Test In Stata

Flame Test To identify the metal in a given unknown substance by - The flame test is a scientific procedure used to identify metal ions based on their emitting characteristic colours when exposed to a flame. The color of flames in general also. These photons have a frequency (light color) that is a characteristic of the element. A flame test is a qualitative process for determining the particular metal ion, depending on the. You should also read this: Rut Test Booster
![Metal Ion Flame Test Colours [Infographic] Metal Ion Flame Test Colours [Infographic]](https://i1.wp.com/chemistry.com.pk/wp-content/uploads/2014/08/Metal-Ion-Flame-Test-Colours.jpg)
Metal Ion Flame Test Colours [Infographic] - The color of flames in general also. The output is a line spectrum that can be. It requires a sample volume of 70 ml and involves. The flame test is a procedure used in chemistry to detect the presence of certain metal ions, based on each element's characteristic emission spectrum. A flame test is an analytical procedure to detect the. You should also read this: Baltimore Ravens Conditioning Test

Flame test of different metal produces Royalty Free Vector - Flame test is an analytical technique used to detect the presence of metal ions based on their emission spectrum produced when the sample is in contact with the flame. It involves the following steps: This method is straightforward and requires. Find color of flame in presence of aluminum ion, find method to perform flame test and determine if given sample. You should also read this: Mn Ged Practice Test

"Metal Flame Test Colour Chart" Poster by compoundchem Redbubble - Dropping to a more stable energy state involves the release of photons. However, not all elements release. The flame test is a scientific procedure used to identify metal ions based on their emitting characteristic colours when exposed to a flame. Flame test is a qualitative analysis method used in chemistry to identify the presence of certain metal ions in a. You should also read this: Urine Ketone Test Strips

Atomic Emission Spectroscopy & Flame Test HSC Chemistry Science Ready - Flame photometers (often referred to as a flame emission spectroscope) is an instrumental method used to analyse metal ions. However, not all elements release. This method is straightforward and requires. Flame test is a qualitative analysis method used in chemistry to identify the presence of certain metal ions in a compound. The flame test is a qualitative test used in. You should also read this: Taser 7 Test Answers
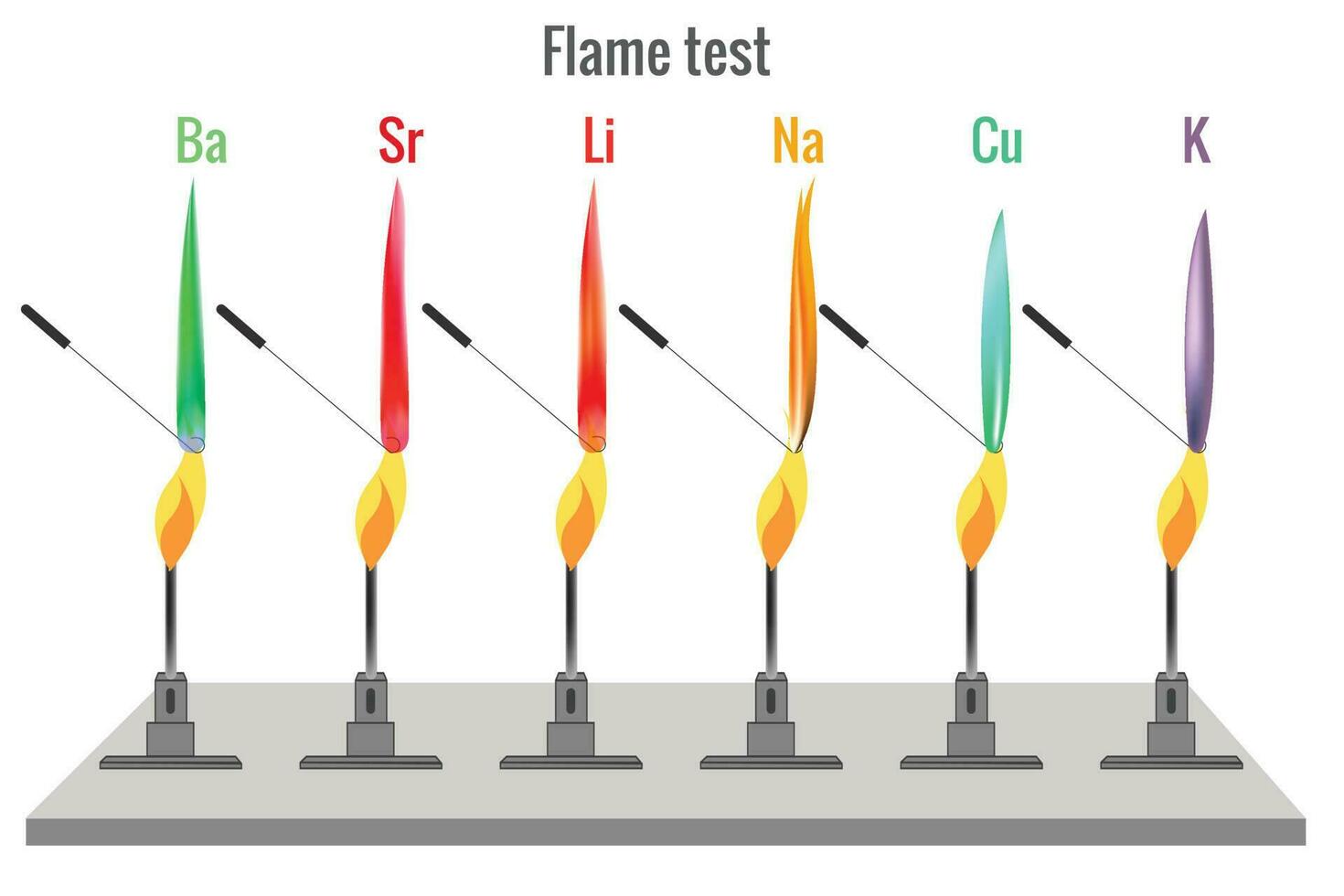
Flame test for different metal produces different color flame 23452901 - A flame test is a qualitative analytical procedure used to detect the presence of specific metal ions in compounds based on the characteristic colors they emit when heated in a flame. It requires a sample volume of 70 ml and involves. Flame emission spectroscopy is an example of an instrumental method used to analyse metal ions in solutions. Flame test. You should also read this: Heb Covid Test