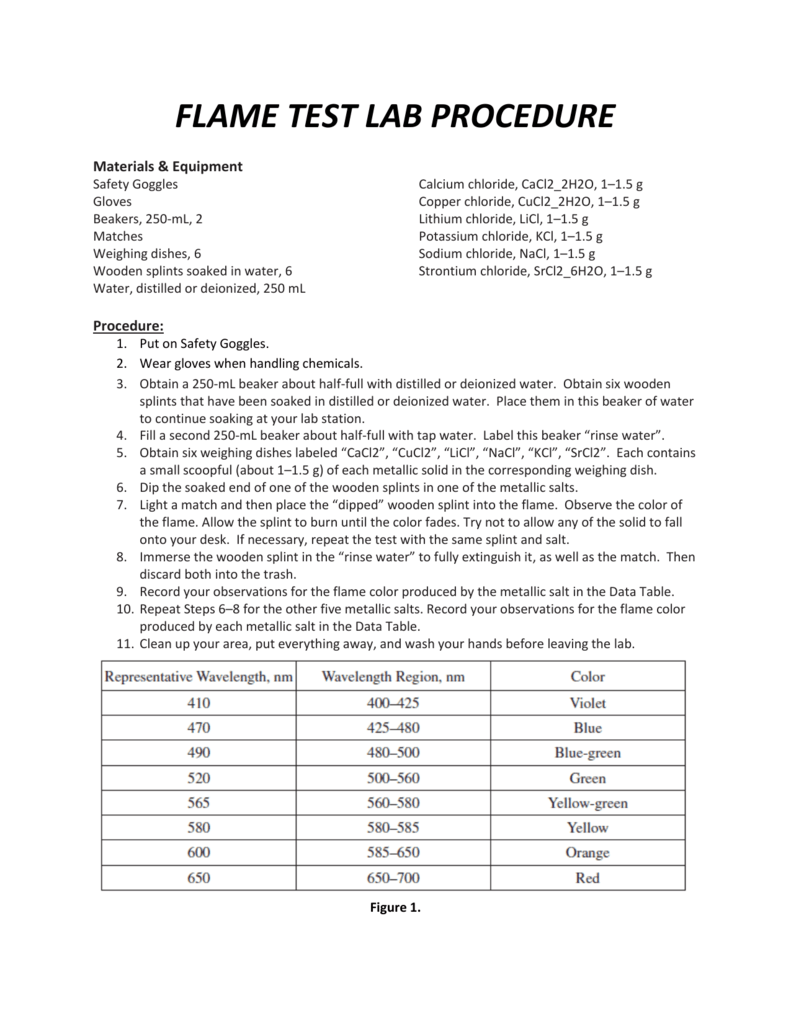
FLAME TEST LAB PROCEDURE - A flame test uses the concept of electron excitation to identify an element. When the boric acid was in the flame, you probably notice a. Conduct flame tests of given salts. The flame test is a safer version of the traditional rainbow demonstration, an exercise popularly conducted in chemistry classrooms. These photons have a frequency (light color) that is a. You should also read this: Akme Drug Testing-quest Diagnostics

Flame Tests Chemistry Practicals YouTube - What is a flame test? These photons have a frequency (light color) that is a characteristic of the element. If the flame color of the metallic elements are. You can use a flame test to help identify the composition of a sample. The objectives of this lab are to: You should also read this: How Do You Test Platinum

Flame Test Lab Chemistry Classes / Ronald Reagan S.H.S. - Chemistry document from liberty university online academy, 5 pages, name libby criscitello date 11/4/24 flame test lab experimental report sheet save this document. Conduct flame tests of given salts. From your results, you will draw conclusions about the colors. What is a flame test? You can use a flame test to help identify the composition of a sample. You should also read this: Litmus Test Synonyms
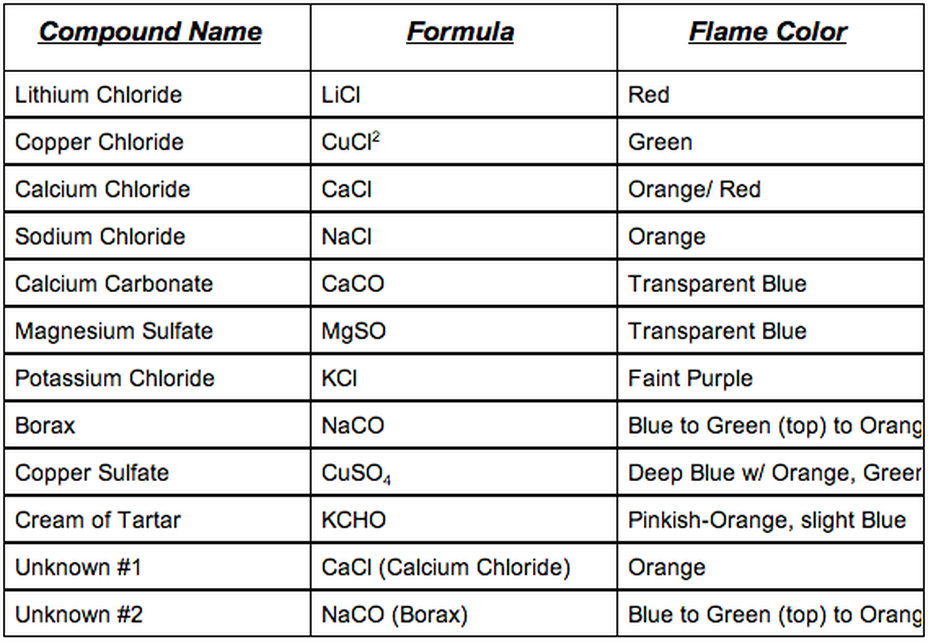
Flame Test Lab Faith's DP - Chemists began studying colored flames in the 18th century and soon used flame tests to distinguish between some elements. If the flame color of the metallic elements are. All of these compounds are flammable, meaning they burn. When energy is supplied to an atom, its electrons. The objectives of this lab are to: You should also read this: Udacity A/b Testing

Amy Brown Science Flame Tests A Favorite Chemistry Lab - Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and. Collin college department of chemistry objectives: When the boric acid was in the flame, you probably notice a. The purpose of the flame test is to demonstrate to. Chemists began studying colored flames in the 18th century and soon used. You should also read this: Sternum Rub Test

Amy Brown Science Flame Tests A Favorite Chemistry Lab - The objectives of this lab are to: When energy is supplied to an atom, its electrons. In this virtual investigation you will burn a small amount of various metal salt solutions and record the colors of the flames. Is the light produced in a flame test. Its purpose is to identify specific elements in a material. You should also read this: Fingerprint Drug Test Detection Times
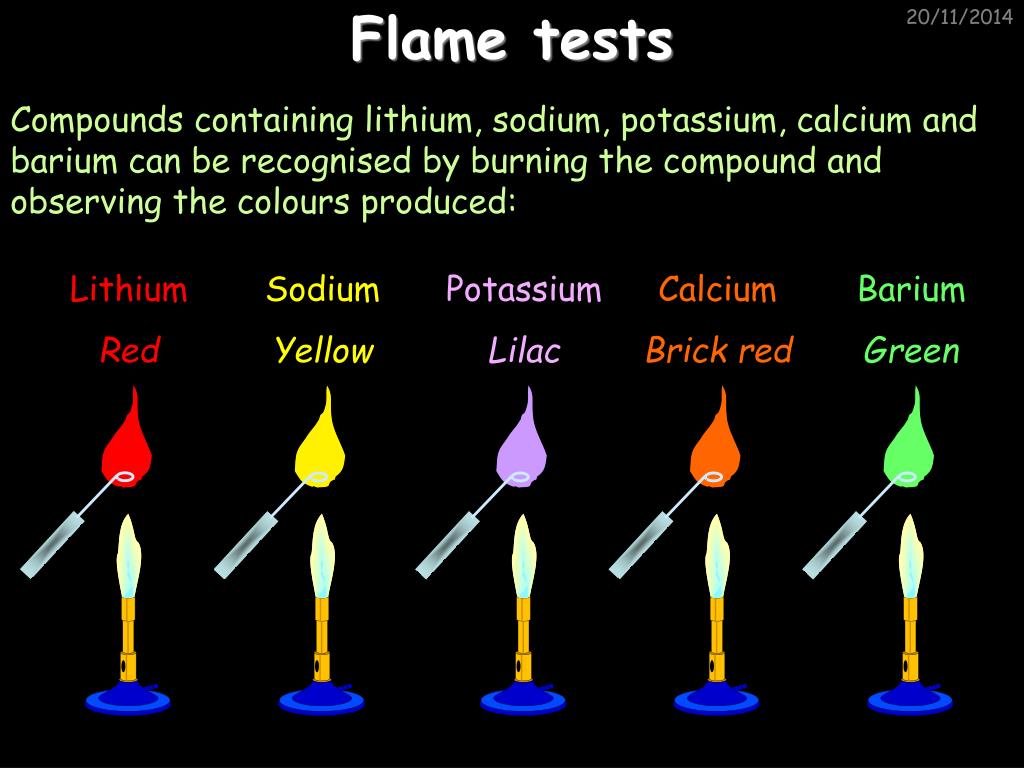
PPT Chemistry in Action PowerPoint Presentation, free download ID - It is widely used to detect and analyze the presence of certain elements in the given. This activity is called a flame test and it’s a real procedure used in labs. Its purpose is to identify specific elements in a material. When metal salts (ionic compounds) are heated in a bunsen burner flame, the metal gives off a characteristic color.. You should also read this: Jiffy Lube Diagnostic Test Cost

Chemistry Flame Test Lab Science Lessons That Rock - A flame test uses the concept of electron excitation to identify an element. Atomic emission spectra are unique patterns of light emitted by elements when their electrons return to lower energy levels after excitation. They found that elements burned with different colored. The flame test is a safer version of the traditional rainbow demonstration, an exercise popularly conducted in chemistry. You should also read this: Cpat Test Ky

Flame Test Video Lab AP Chemistry 1415 Final YouTube - Chemistry document from liberty university online academy, 5 pages, name libby criscitello date 11/4/24 flame test lab experimental report sheet save this document. Conduct flame tests of given salts. This activity is called a flame test and it’s a real procedure used in labs. What does the law of conservation of energy have to do with this type of experiment?. You should also read this: 8v Battery Load Tester

Flame Test Lab YouTube - The test is performed by dipping a wire or wooden splint into a sample solution or coating it with the powdered metal salt. All of these compounds are flammable, meaning they burn. However, not all elements release. In your own words, explain how the colors observed in the flame tests are produced and why different ions will produce different colors. You should also read this: Define Split Testing