
Aspirin Purity Test Ferric Chloride YouTube - Learn how to make aspirin from salicylic acid and acetic anhydride, and test its purity with iron(iii) chloride solution. Be as specific as possible. What precautions should one use when working. As with most tests, it is not perfect and some phenols do. The ferric chloride test results showed that prepared aspirin and salicylic acid yielded a violet solution, indicating. You should also read this: Christmas Light String Tester

Ferric Chloride Test for Phenols YouTube - Ferric chloride test for salicylic acid. Ferric chloride reagent is used as a colorimetric test for phenols: More soluble in ethanol specific. What is used in ferric chloride test? However, even very small amounts of a salicylate, such as a single ingested aspirin. You should also read this: Does Quest Process Blood Tests On The Weekends

Solved Result of the ferric chloride test on aspirin light - Why is the aspirin washed with cold water? Ferric chloride test for salicylic acid. Ferric chloride test results the ferric chloride test is commonly used to detect the presence of salicylates, including acetylsalicylic acid, which is the active ingredient in aspirin. What is used in ferric chloride test? What precautions should one use when working. You should also read this: Test De Alcohol Y Drogas Florida En Español Respuestas
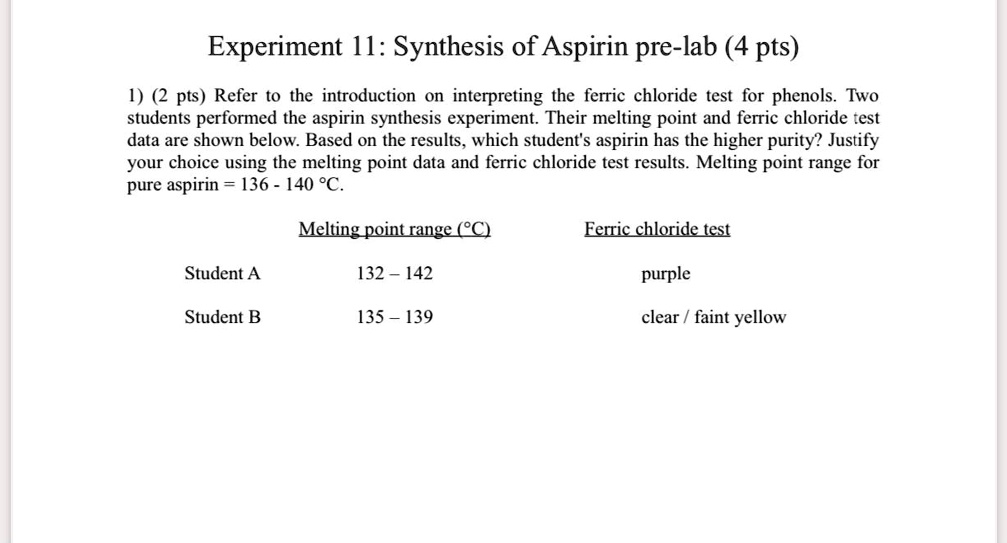
experiment i synthesis of aspirin pre lab 4 pts 1 2 pts refer to the - More soluble in ethanol specific. Be as specific as possible. Physical test of aspirin color: Describe how and why the ferric chloride test can be used to detect whether your reaction has gone to completion. Ferric chloride reagent is used as a colorimetric test for phenols: You should also read this: Ap Environmental Science Unit 8 Test
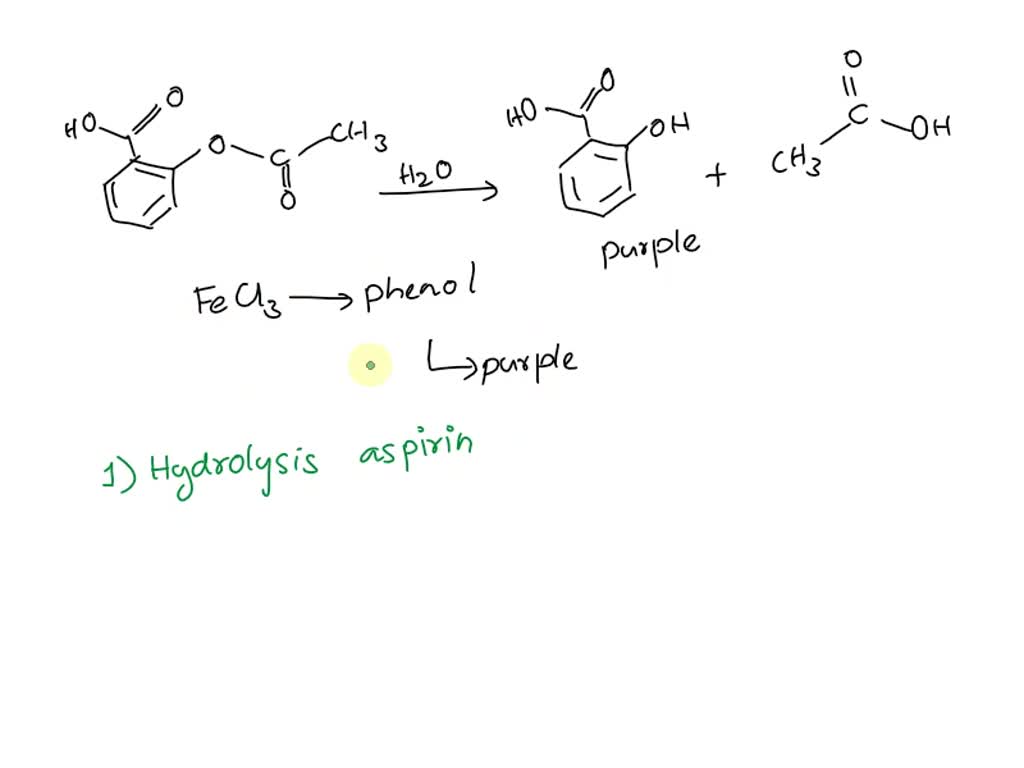
SOLVED Ferric Chloride Test A purpleish color suggests the presence - According to your results from the ferric chloride test, what can you say about the purity of your aspirin? The purity of the synthesized product can be tested with ferric chloride, fecl3. You will synthesize aspirin and test its purity using the ferric chloride test. If any salicylic acid remains, it would form a colored complex with ferric chloride, resulting.. You should also read this: Binaxnow Covid Test Extension
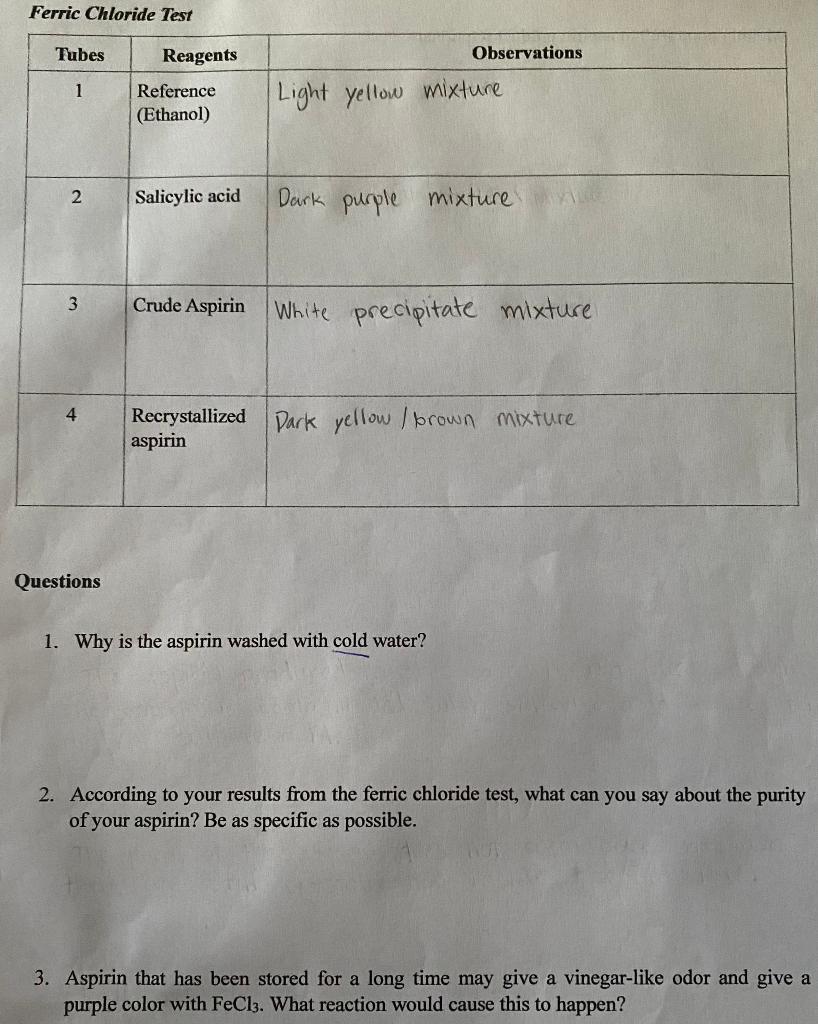
Solved Ferric Chloride Test 2uestions 1. Why is the aspirin - More soluble in ethanol specific. What is used in ferric chloride test? After the addition of the ferric chloride, the salicylic acid solution turned purple,. Positive results with the urine ferric chloride test only indicate that a salicylate is present; Briefly explain the purpose of the fecl3 test. You should also read this: Car Battery Test Results Cca

Ferric Chloride Test ANSWER TO QUESTIONS Physical test of aspirin - Add 10 drops of aqueous 1% ferric chloride solution to a test tube containing a few crystals of the compound to be tested dissolved in 5. Ferric chloride test for salicylic acid. The ferric chloride test results showed that prepared aspirin and salicylic acid yielded a violet solution, indicating the presence of phenols. As with most tests, it is not. You should also read this: Cystatin C Test Normal Range

Synthesis of Aspirin Experiment Six. ppt download - Briefly explain the purpose of the fecl3 test. What is used in ferric chloride test? Add 10 drops of aqueous 1% ferric chloride solution to a test tube containing a few crystals of the compound to be tested dissolved in 5. The fe ion reacts with the phenol group of salicylic acid to give a purple color. Add a few. You should also read this: American Temperament Test Society Top 10

What Happens When Aspirin/Salicylic Acid Is Treated with Ferric - The purity of the synthesized aspirin can be tested by addition of fe+3 to a suspension of the product. Positive results with the urine ferric chloride test only indicate that a salicylate is present; Phenols (such as salicylic acid) react with ferric chloride to form colored You will synthesize aspirin and test its purity using the ferric chloride test. What. You should also read this: Valgus Varus Stress Test
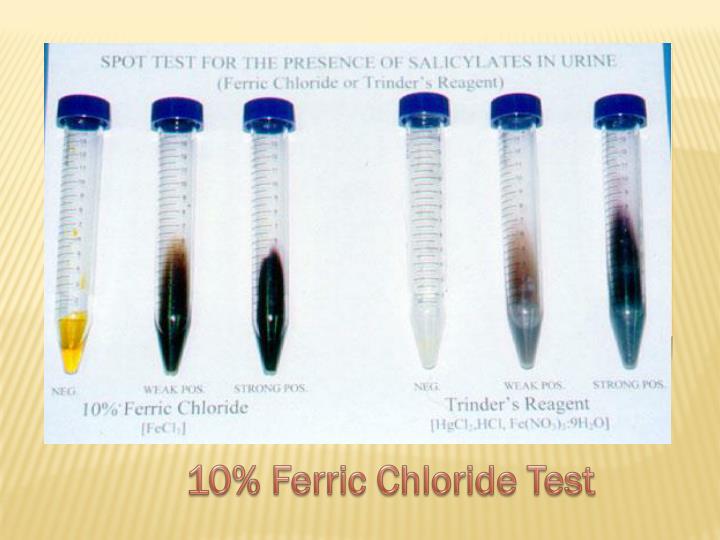
PPT Highlights in Pediatrics Toxicology PowerPoint Presentation ID - Ferric chloride test results the ferric chloride test is commonly used to detect the presence of salicylates, including acetylsalicylic acid, which is the active ingredient in aspirin. What precautions should one use when working. After the addition of the ferric chloride, the salicylic acid solution turned purple,. What does a positive fecl3 test signify? Ferric chloride test for salicylic acid. You should also read this: Dmv Practice Test In Creole