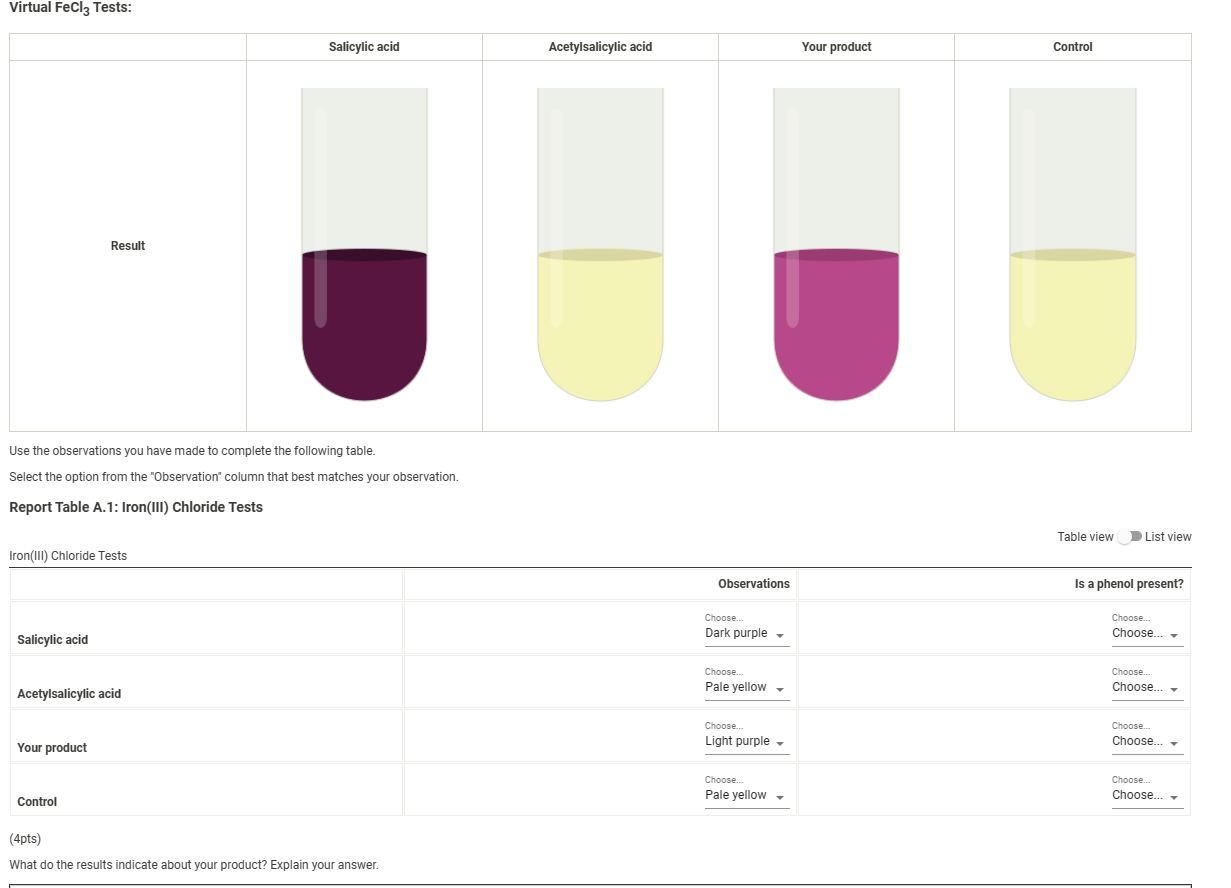
Solved Virtual FeCl3 Tests Use the observations you have - Separately test each of the following in a test tube, with 1 ml of ethanol and 3 drops of 0.02 m aq. Aspirin that has been stored for a long time may give a. The ferric chloride test is a qualitative analysis method used to determine the presence of phenolic compounds, specifically salicylic acid, in a sample. The starch test. You should also read this: Road Test In Yonkers

result and discussion of fecl3 and starch test wi aspirin Organic - The starch test showed that prepared aspirin yielded a negative result, while commercially available aspirin and the control yielded positive results, suggesting the presence of starch. Any unreacted salicylic acid will cause a solution of the synthesized aspirin to turn purple when iron (iii) chloride is added. Separately test each of the following in a test tube, with 1 ml. You should also read this: 5 Strands Allergy Test
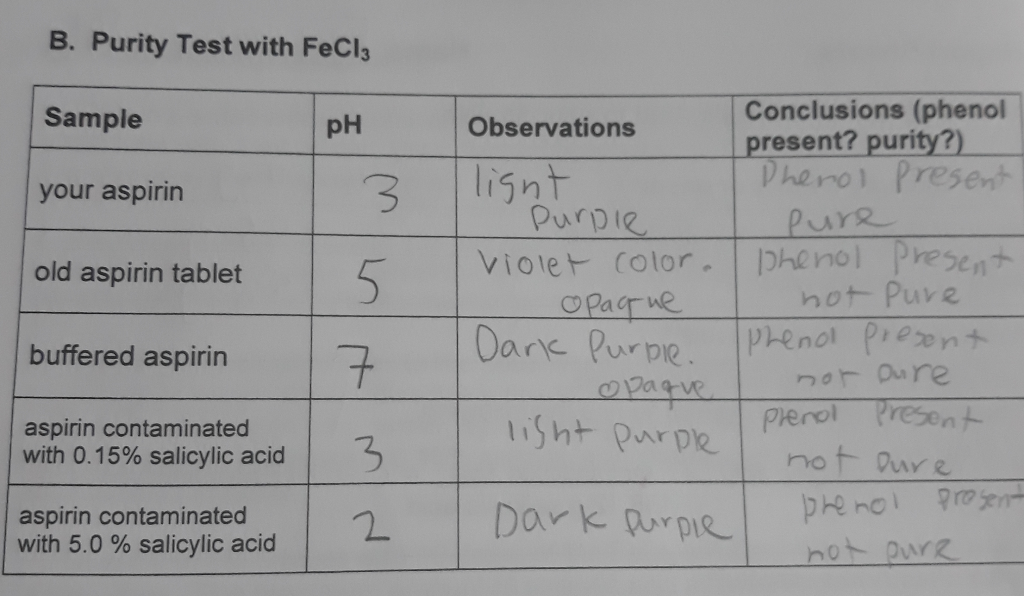
Solved We determined the purity of the aspirin we - The purity of aspirin can be tested using fecl (aq) as it reacts with phenols to form a purple complex. Iron (iii) ion reacts with phenols to form a purple complex. Separately test each of the following in a test tube, with 1 ml of ethanol and 3 drops of 0.02 m aq. Aspirin that has been stored for a. You should also read this: How Many Times Can You Reheat Urine For Drug Test
[Solved] I need help with my synthesis of Aspirin Lab. mission - If phenols react with fecl3 (aq.) to give a positive result (color change), which compound (s) in the reaction above do you predict will give a positive test? The purity of the synthesized aspirin can be tested by addition of fe+3 to a suspension of the product. Mix several drops or a few crystals of compound to be tested in. You should also read this: Primary Ciliary Dyskinesia Testing

Aspirin Purity Test Ferric Chloride YouTube - Commercially prepared aspirin may also give a positive iron (iii). Aspirin does not contain a phenol group, so it will not react with fecl3. Mix several drops or a few crystals of compound to be tested in a beaker or in a 200mm test tube. The starch test showed that prepared aspirin yielded a negative result, while commercially available aspirin. You should also read this: Modern Rice Purity Test

Synthesis of Aspirin Experiment Six. ppt download - Fecl3 is used to indicate the presence of a phenol group by producing a purple color. The ferric chloride test was used to compare the salicylic acid, crude aspirin, and purified aspirin. Aspirin that has been stored for a long time may give a. Salicylic acid contains a phenol group, but acetylsalicylic acid (aspirin). Salicylic acid contains a phenol group,. You should also read this: Can You Use Someone Else's Car For Driving Test
![Solved] Fecl3 Reacts With Only One Of The Following, 44 OFF Solved] Fecl3 Reacts With Only One Of The Following, 44 OFF](https://cdn.numerade.com/ask_previews/75e161d3-6dee-44fa-ad12-afd9eafe6976_large.jpg)
Solved] Fecl3 Reacts With Only One Of The Following, 44 OFF - If aspirin reacts with fecl3 to form a purple color, it means the sample is impure and contains salicylic acid. Iron (iii) ion reacts with phenols to form a purple complex. Would aspirin test positive for phenol? Commercially prepared aspirin may also give a positive iron (iii). The purity of the synthesized aspirin can be tested by addition of fe+3. You should also read this: Anime Like Baka And Test
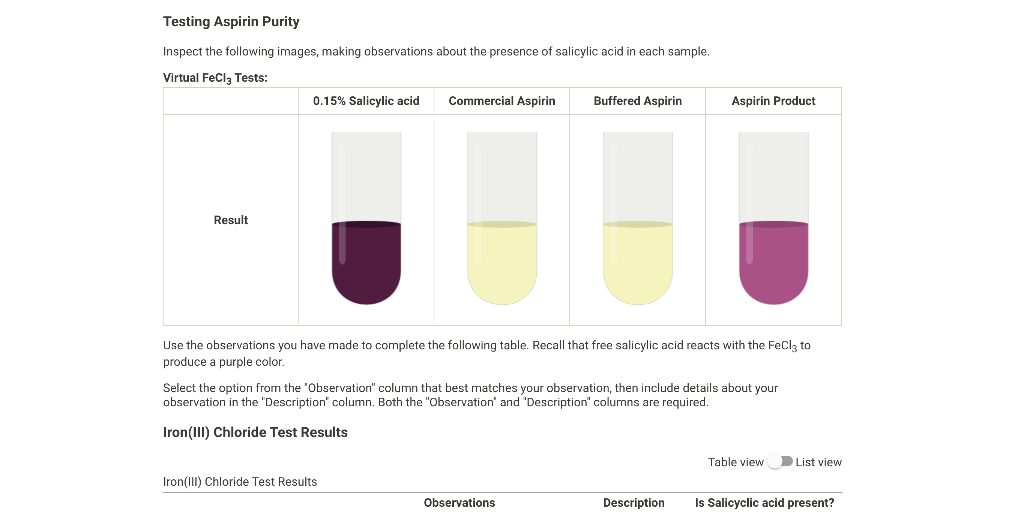
Solved Testing Aspirin Purity Inspect the following images, - The ferric chloride test is a qualitative analysis method used to determine the presence of phenolic compounds, specifically salicylic acid, in a sample. The purity of aspirin can be tested using fecl (aq) as it reacts with phenols to form a purple complex. Would aspirin test positive for phenol? Mix several drops or a few crystals of compound to be. You should also read this: Can Sudafed Make You Fail A Drug Test
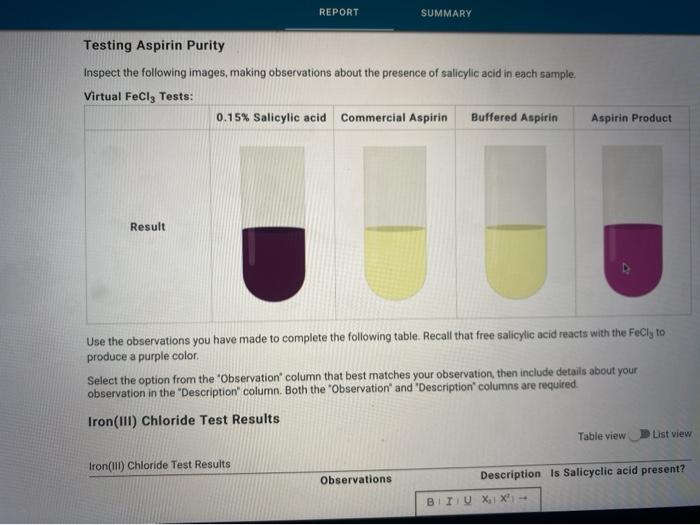
Solved REPORT SUMMARY Testing Aspirin Purity Inspect the - We are testing a portion of the synthesized aspirin with fecl3 to establish whether salicyclic acid behaves as a phenolic alcohol or as a carboxylic acid. The collected aspirin will be tested for its purity using fecl3 (aq). Commercially prepared aspirin may also give a positive iron (iii). Add a few drops of 1%. In this experiment, the crystalline product. You should also read this: Strep Throat Test False Positive
SOLVED Which of the following is used to test for the purity of - Iron (iii) ion reacts with phenols to form a purple complex. The collected aspirin will be tested for its purity using fecl3 (aq). According to your results from the ferric chloride test, what can you say about the purity of your aspirin? The starch test showed that prepared aspirin yielded a negative result, while commercially available aspirin and the control. You should also read this: Sluggish Cognitive Tempo Test