What Blood Test Is Used For Colon Cancer at Everett Gamboa blog - The united states food and drug administration (fda) approved a new blood test to screen for colon cancer on july 29. In october, the fda approved shield by guardant, which looks for differences in the blood, including cancer dna that might suggest colorectal cancer. Food and drug administration (fda). Food and drug administration (fda) approved guardant health’s crc blood test,. You should also read this: Iready Test Score Meaning

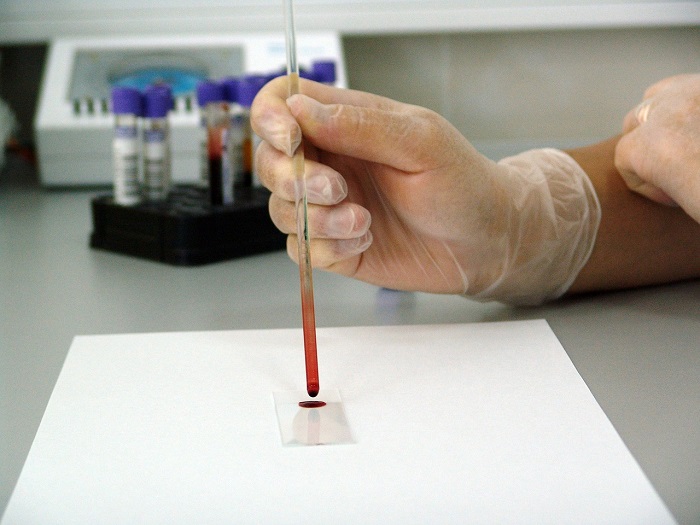
Reduce Your Risk of Colorectal Cancer Healthwise Foods - The food and drug administration approved a blood test intended to detect colorectal cancer on monday, expanding options for screening for the potentially deadly disease. Noninvasive & effectivepatient siteeasy to use24/7 patient support Food and drug administration (fda) voted in favor of approving the test, per everyday health’s network site, medpage today. The fda has approved a new blood test. You should also read this: Johnson And Johnson Senior Test Engineer Interview Questions

Reduce Your Risk of Colorectal Cancer Healthwise Foods - In october, the fda approved shield by guardant, which looks for differences in the blood, including cancer dna that might suggest colorectal cancer. An estimated 154,270 people in the u.s. The test isn't meant to replace colonoscopies,. Food and drug administration on monday approved a new blood test that can spot colon cancer. The fda approved the shield blood test. You should also read this: Best Crepe Pan America's Test Kitchen
What Blood Test Is Used For Colon Cancer at Everett Gamboa blog - Shield is now covered by. Food and drug administration (fda) approved guardant health’s crc blood test, shield, which scans for signs of cancer in adults aged 45 and over. The fda granted marketing authorization of the first diagnostic test for chlamydia, gonorrhea and trichomoniasis that can be purchased without a prescription and performed. On july 29, 2024, the fda approved. You should also read this: Perc Tested Meaning
What Blood Test Is Used For Colon Cancer at Everett Gamboa blog - Food and drug administration on monday approved a new blood test that can spot colon cancer. Early crc detectionscreen eligible patientstelehealth availableuspstf recommended The test from guardant health called shield is not. On july 29, 2024, the fda approved shield blood test for primary colorectal cancer screening. Test manufacturer guardant said the food and drug administration approved its shield test. You should also read this: Light Test Mtg

FDA approves blood test for colorectal cancer screening among average - The food and drug administration on monday approved guardant health’s blood test, called shield, to screen for colon cancer. On july 29, 2024, the fda approved shield blood test for primary colorectal cancer screening. On may 23, an advisory panel for the u.s. Food and drug administration (fda) approved guardant health’s crc blood test, shield, which scans for signs of. You should also read this: Shoulder Anterior Drawer Test
:quality(70)/cloudfront-us-east-1.images.arcpublishing.com/cmg/UGTDSVI4NZAT5HYGLIDILTIFTI.jpeg)
FDA approves colorectal cancer blood test WSBTV Channel 2 Atlanta - The food and drug administration approved a blood test intended to detect colorectal cancer on monday, expanding options for screening for the potentially deadly disease. On may 23, an advisory panel for the u.s. Shield, made by guardant health, is the first approved blood test that is considered a primary screening option. The fda has approved a new blood test. You should also read this: Dmv El Cajon Driving Test Route

F.D.A. Approves Blood Test for Colon Cancer Detection The New York Times - In october, the fda approved shield by guardant, which looks for differences in the blood, including cancer dna that might suggest colorectal cancer. Noninvasive & effectivepatient siteeasy to use24/7 patient support Community advocacy groupstips & remindersresources & support Test manufacturer guardant said the food and drug administration approved its shield test for screening in adults 45 and older who have. You should also read this: What Blood Test For Leukemia

FDA approves new blood test to screen for colon cancer NBC Bay Area - The test isn't meant to replace colonoscopies,. In october, the fda approved shield by guardant, which looks for differences in the blood, including cancer dna that might suggest colorectal cancer. The test from guardant health called shield is not. The food and drug administration on monday approved guardant health’s blood test, called shield, to screen for colon cancer. Food &. You should also read this: Schirmer Test Strips

New blood test could boost colon cancer screening rates in Texas CBS - The food and drug administration approved a blood test intended to detect colorectal cancer on monday, expanding options for screening for the potentially deadly disease. The food and drug administration on monday approved guardant health’s blood test, called shield, to screen for colon cancer. On july 29, 2024, the fda approved shield blood test for primary colorectal cancer screening. In. You should also read this: Pj Testa Accountant Tampa