
FDA Clears Test for Early Sepsis Detection Respiratory Therapy - The fda approval of intellisep, the first sepsis diagnostic tool, could change this. Abionic, an emerging medical diagnostics company focused on rapid early detection technologies, received 510(k) clearance for its ivd capsule psp test from the u.s. Triverity is the first test to provide rapid infection scoring and illness severity evaluation for suspected acute infections or sepsis. The test measures. You should also read this: My Doctor Won T Call Me Back With Test Results

OSF HealthCare research contributes to FDA approval of AI sepsis tool - Today, cytovale announced their early sepsis diagnostic test, intellisep, received us food. With results in about 30 minutes, the benchtop triverity test is designed to help emergency department clinicians determine whether a patient is fighting off a bacterial or viral infection. The ivd capsule psp test aims to address these challenges by detecting sepsis earlier than current diagnostic methods. Triverity. You should also read this: Vintage Electronic Test Equipment

Predictive Biomarkers Brighten the Precision - The us food and drug administration (fda) has approved a medical device named the sepsis immunoscore, which is an artificial intelligence/machine learning software, to guide rapid diagnosis and prediction of sepsis. The triverity test provides rapid measurement of infection likelihood and risk, triaging patients with acute infections and sepsis, and quickly sorting patients into a ‘grey zone’. Triverity is the. You should also read this: How To Keep Urine Warm For Test

JCM Free FullText Clinical Utility of Recently Food and Drug - Intellisep assesses cellular host response via deformability cytometry of leukocyte biophysical properties and intended for use in conjunction with clinical assessments and. Deepull also recently received breakthrough device designation from the us fda for a sepsis test that is used to detect pathogens directly from blood. With results in about 30 minutes, the benchtop triverity test is designed to help. You should also read this: Culpepper Testing Laboratories

JCM Free FullText Clinical Utility of Recently Food and Drug - Triverity is the first test to provide rapid infection scoring and illness severity evaluation for suspected acute infections or sepsis. The fda approval of intellisep, the first sepsis diagnostic tool, could change this. The triverity test provides rapid measurement of infection likelihood and risk, triaging patients with acute infections and sepsis, and quickly sorting patients into a ‘grey zone’. This. You should also read this: 126 Iq Test

AI Sepsis Diagnostic Tool Gets FDA Authorization MedPage Today - With results in about 30 minutes, the benchtop triverity test is designed to help emergency department clinicians determine whether a patient is fighting off a bacterial or viral infection. Lactate is a recognized biomarker for tissue hypoperfusion and organ. Abionic, an emerging medical diagnostics company focused on rapid early detection technologies, received 510(k) clearance for its ivd capsule psp test. You should also read this: Tesla Test Drive Tampa
![FDA approved protein biomarkers [3,5,14,15]. Download Scientific Diagram FDA approved protein biomarkers [3,5,14,15]. Download Scientific Diagram](https://www.researchgate.net/publication/344225827/figure/tbl1/AS:936072506798080@1600188485127/FDA-approved-protein-biomarkers-3-5-14-15.png)
FDA approved protein biomarkers [3,5,14,15]. Download Scientific Diagram - With results in about 30 minutes, the benchtop triverity test is designed to help emergency department clinicians determine whether a patient is fighting off a bacterial or viral infection. Today, cytovale announced their early sepsis diagnostic test, intellisep, received us food. Deepull also recently received breakthrough device designation from the us fda for a sepsis test that is used to. You should also read this: Echelon Prevention Of Medical Errors Post Test
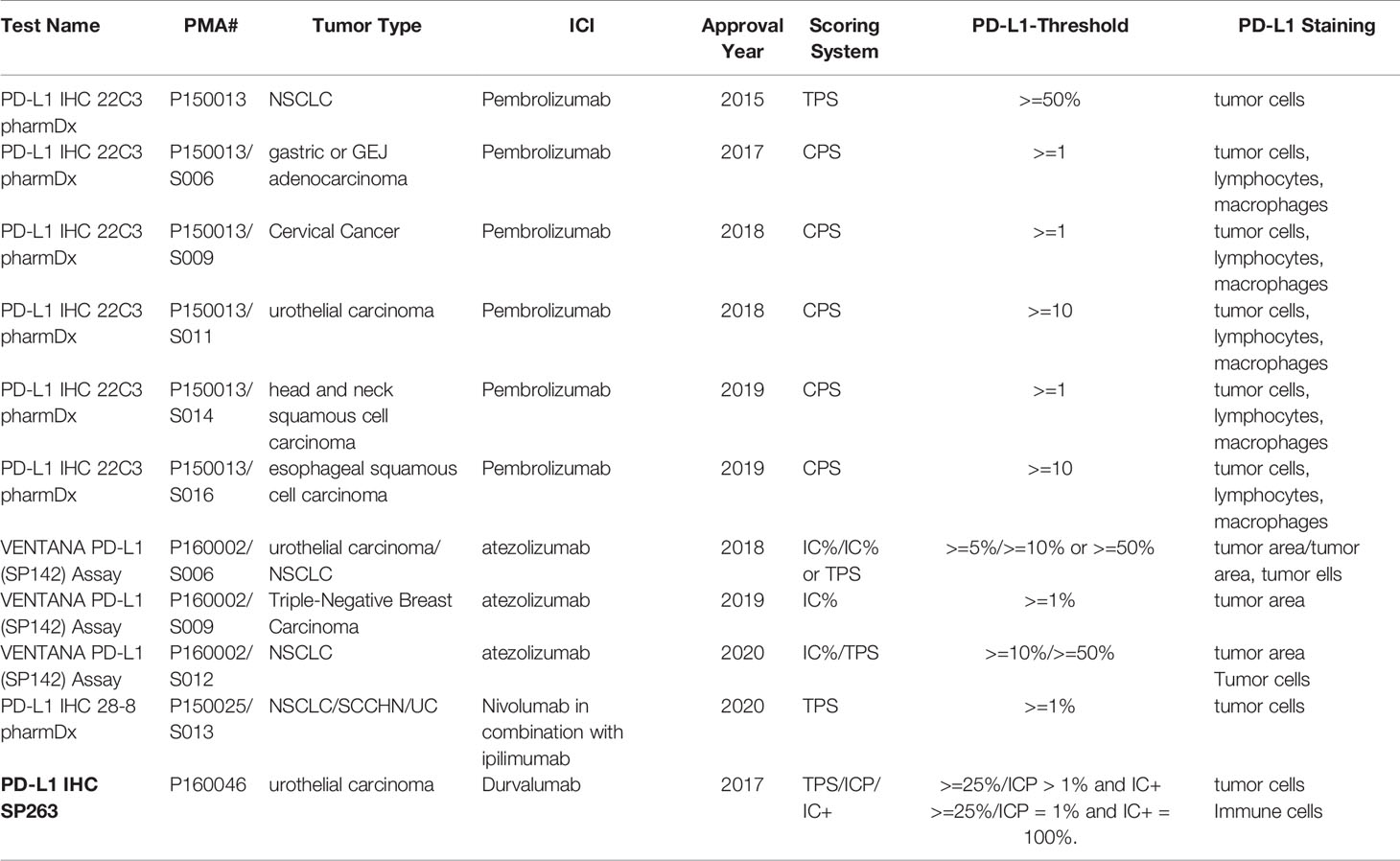
Frontiers FDAApproved and Emerging Next Generation Predictive - Triverity is the first test to provide rapid infection scoring and illness severity evaluation for suspected acute infections or sepsis. Intellisep is the first and only test fda cleared for sepsis detection. Today, cytovale announced their early sepsis diagnostic test, intellisep, received us food. This highlights the need for readily available and reliable biomarkers to guide the management of sepsis.. You should also read this: Ellume Covid Test App Download

(PDF) Clinical Utility of Recently Food and Drug Administration - This highlights the need for readily available and reliable biomarkers to guide the management of sepsis. The triverity test provides rapid measurement of infection likelihood and risk, triaging patients with acute infections and sepsis, and quickly sorting patients into a ‘grey zone’. The firm unveiled the ullcore bsi test this spring along with the company's flagship ullcore molecular testing platform.. You should also read this: Kvcc Testing Center

Biomarker categories according to the BEST resource (FDA) Download - The us food and drug administration (fda) has approved a medical device named the sepsis immunoscore, which is an artificial intelligence/machine learning software, to guide rapid diagnosis and prediction of sepsis. Food and drug administration approved an ai tool that can diagnose sepsis, prenosis, the company behind the software, announced wednesday, the latest in a. Today, cytovale announced their early. You should also read this: Cdl General Knowledge Test Va