
Sterility Testing for Cellular Therapies What Is the Role of the - At lucideon, we use two methods to perform sterility testing: Sterility tests can be carried out using one of the following methods, methods a: There are two recommended methods of sterility testing for pharmaceuticals: In the direct inoculation (immersion) method, the test articles are inoculated directly into tubes or bottles containing an appropriate medium and incubated for 14 days. To. You should also read this: Does Nic Show Up In Drug Test
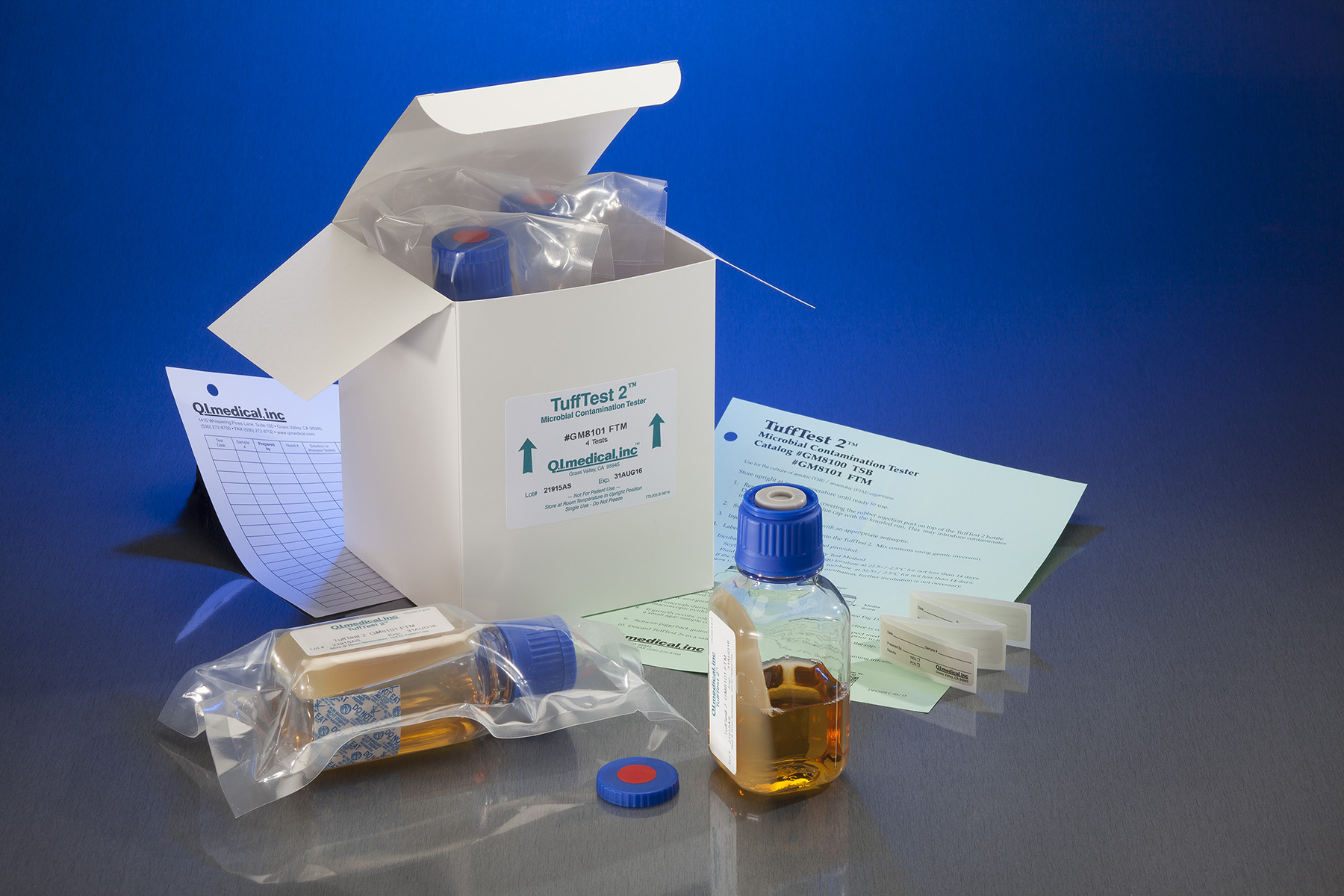
Pharmaceutical Testing Instruments QI Medical, Inc. - Inoculation in two types of media for detection of aerobic and anaerobic microorganisms. Direct inoculation (immersion) in the direct inoculation method the samples are inoculated directly into tubes or bottles that contain an appropriate medium and are incubated for a period of 14. Sterility testing is a critical quality control process for cell and gene therapy products (cgtps), where rapid. You should also read this: Home Depot Radon Test Kit

Sterility TestSolutionsZHEJIANG TAILIN BIOENGINEERING CO.,LTD - Membrane filtration and direct inoculation. All devices, with the exception of devices with pathways labeled sterile, are tested using the direct inoculation of the culture medium method. Discover our full sterility testing portfolio based on over 50 years of experience and expertise. At lucideon, we use two methods to perform sterility testing: Select from our range of sterility testing media. You should also read this: 8dpo First Response Test
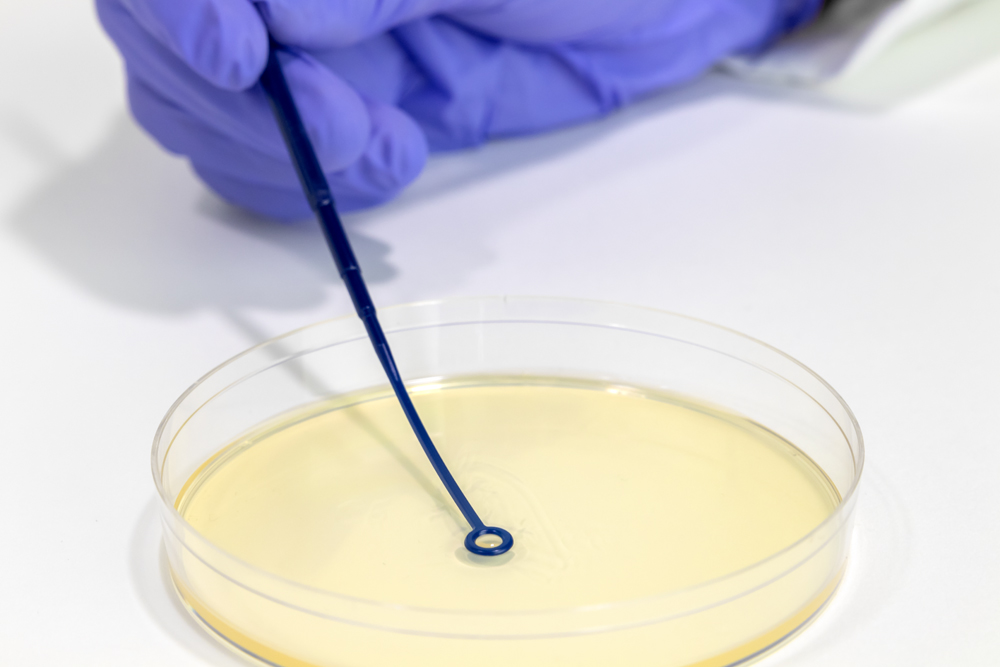
Inoculation Loops and Needles Typenex Medical - Membrane filtration and direct inoculation. Select from our range of sterility testing media including soybean casein digest. In the direct inoculation (immersion) method, the test articles are inoculated directly into tubes or bottles containing an appropriate medium and incubated for 14 days. To comply with your different direct inoculation method requirements, we offer sterility test media in various volumes, from. You should also read this: Smog Test Simi Valley

Paving the Way for Dose Banding of Chemotherapy An Analytical Approach - Direct inoculation (immersion) in the direct inoculation method the samples are inoculated directly into tubes or bottles that contain an appropriate medium and are incubated for a period of 14. Inoculation in two types of media for detection of aerobic and anaerobic microorganisms. Complete sterility testing solutions for complete confidence. Membrane filtration sterility testing is the regulatory method of choice.. You should also read this: Wl Vue Testing Exam
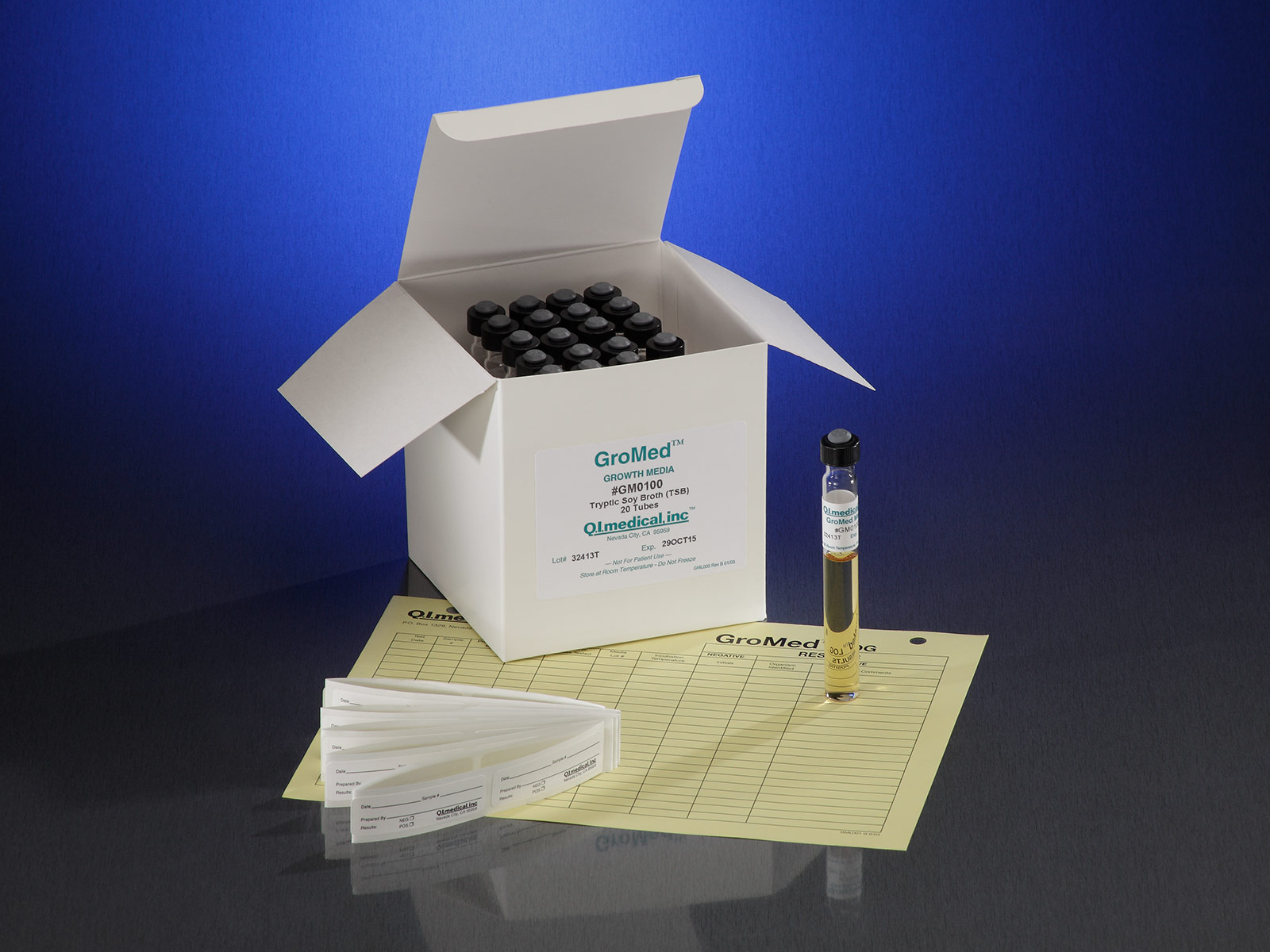
Tubes with TSB Broth for Direct Inoculation QI Medical, Inc. - Inoculation period of 14 days with. By far the most common method is membrane filtration, which involves passing. Inoculation in two types of media for detection of aerobic and anaerobic microorganisms. Membrane filtration sterility testing is the regulatory method of choice. Provisions for retesting are included under. You should also read this: California Rda Practice Test

Sterility Testing Kits Negative/Positive Photos QI Medical, Inc. - Provisions for retesting are included under. Sterility testing is a critical quality control process for cell and gene therapy products (cgtps), where rapid and reliable detection of microbial contaminants is essential to meet regulatory. To comply with your different direct inoculation method requirements, we offer sterility test media in various volumes, from 9 ml tubes up to 750 ml bottles.. You should also read this: The Test Dream The Sopranos

Regulatory Standards for Sterility Testing - Complete sterility testing solutions for complete confidence. All devices, with the exception of devices with pathways labeled sterile, are tested using the direct inoculation of the culture medium method. Sterility testing is a critical quality control process for cell and gene therapy products (cgtps), where rapid and reliable detection of microbial contaminants is essential to meet regulatory. By far the. You should also read this: Which Of The Following Is Not On The Permit Test

Sterilizing Inoculation Loop Photograph by Microgen Images/science - Discover our full sterility testing portfolio based on over 50 years of experience and expertise. Inoculation period of 14 days with. Sterility testing is a critical quality control process for cell and gene therapy products (cgtps), where rapid and reliable detection of microbial contaminants is essential to meet regulatory. In the direct inoculation (immersion) method, the test articles are inoculated. You should also read this: Fbla Computer Problem Solving Practice Test

Sterility test products (and Endotoxin test) Hylabs - Membrane filtration sterility testing is the regulatory method of choice. This is normally conducted on stoppers or other materials that. Sterility tests can be carried out using one of the following methods, methods a: By far the most common method is membrane filtration, which involves passing. To comply with your different direct inoculation method requirements, we offer sterility test media. You should also read this: Ucf Business Testing Center