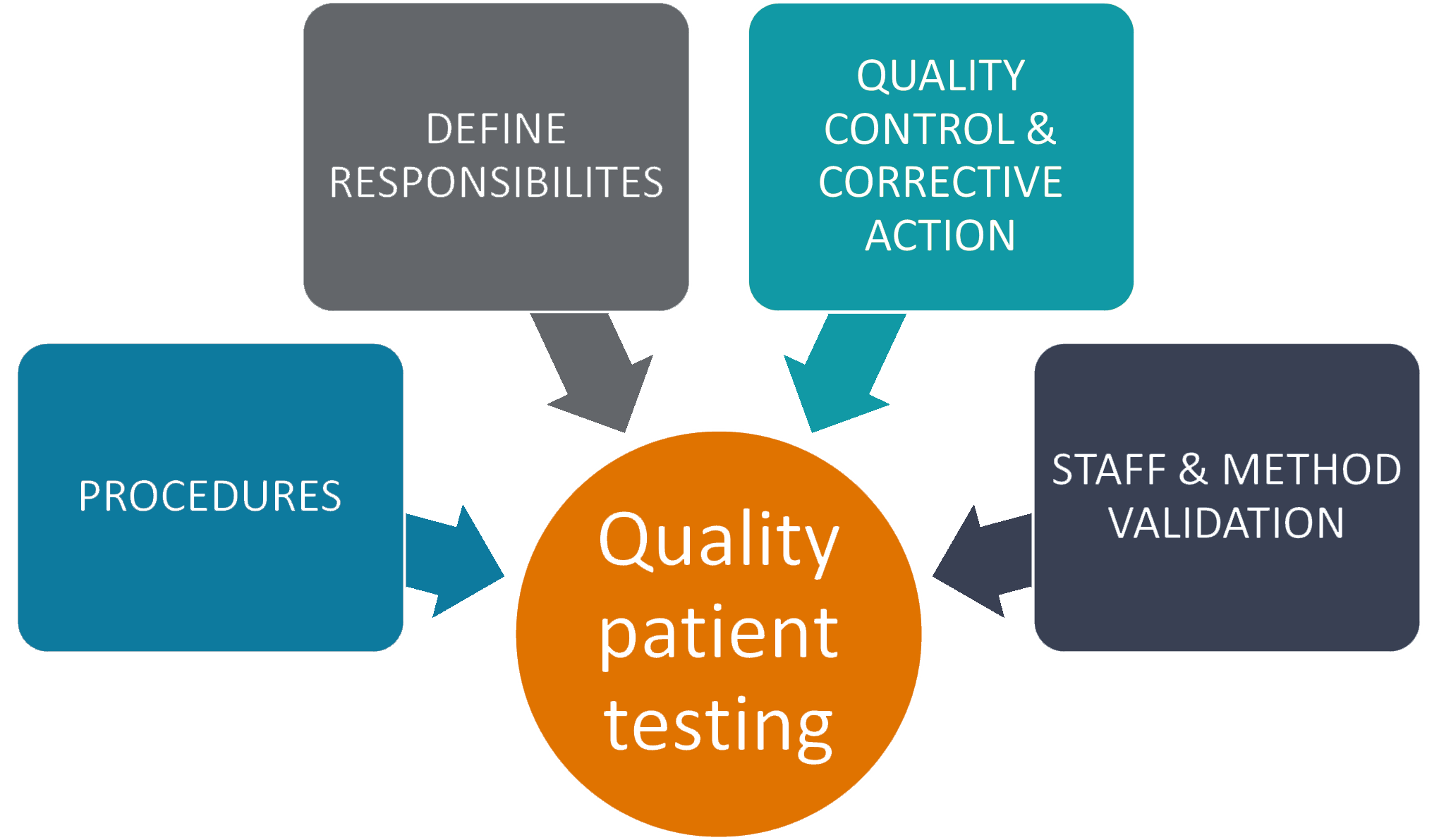
CLIA and Quality Assurance AAFP - Obtaining a clia certificate is a critical step for laboratories that perform testing on human specimens. Notify the physician of critical results and document the two essential steps in following up on a critical value are: Assess the progress of disease. The intent of the clia. • monitor testing and storage areas to assure they meet environmental requirements described in. You should also read this: Scl70 Blood Test
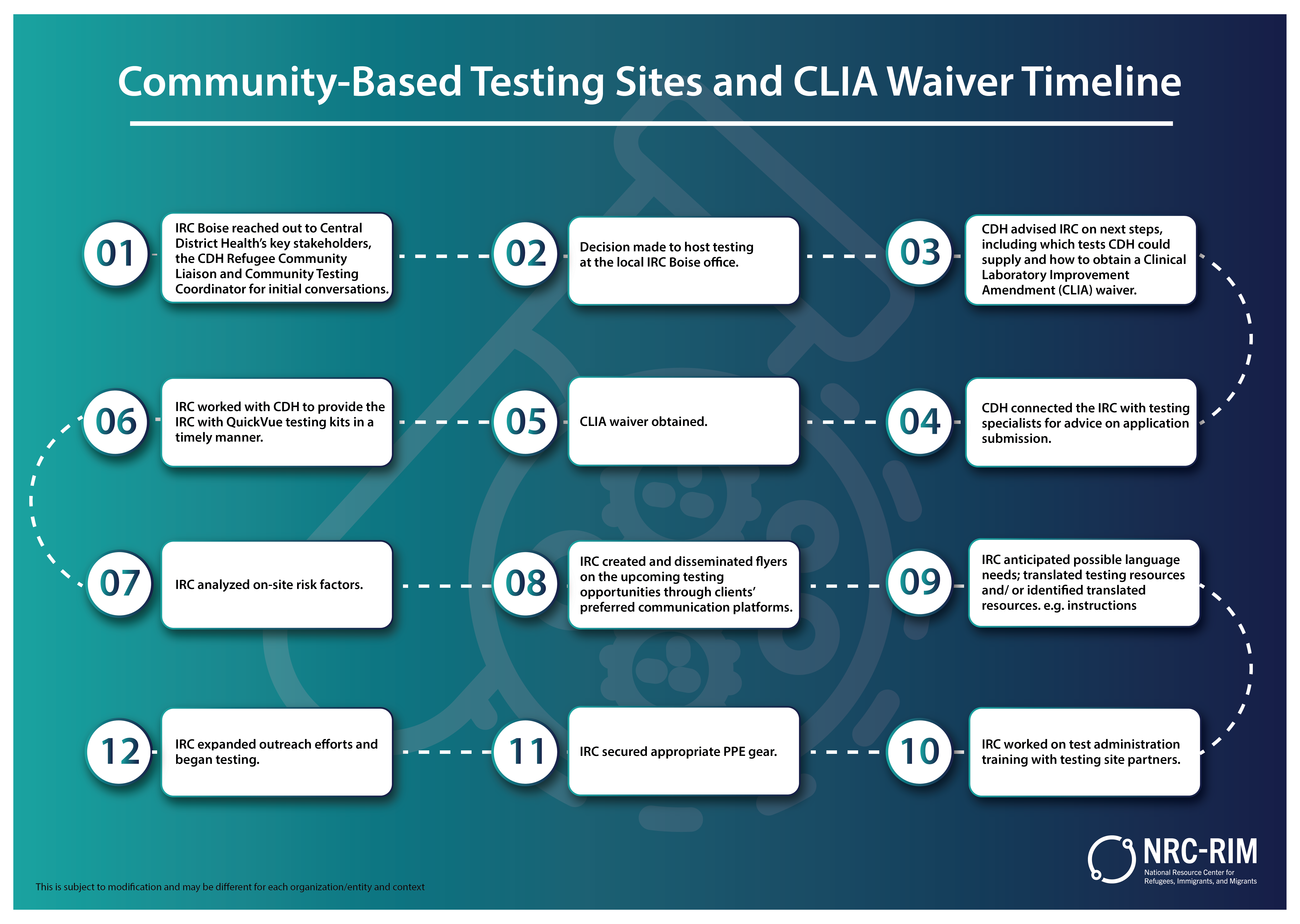
CommunityBased Testing Sites and CLIA Waiver National Resource - The three levels of lab testing defined by clia are: In this post, i will discuss three essential aspects of the analytic phase of testing for amplification assays that your lab needs to pay close attention to in order to ensure high. Obtaining clia certification is a critical step for medical labs and phlebotomy facilities in the united states. Spun. You should also read this: Group Compatibility Test Mbti
Best Practices for Maintaining CLIA Certification and Compliance - Examples of critical steps in clia testing include: What does the clinical laboratory screen? Which of the following is a correct. Spun hematocrit and rapid streptococcus. Confirm condition suspicious by the physicians. You should also read this: Mold Testing Kits Lowes

CLIA Compliance - Which of the following is a correct. Determine whether the laboratory performs only waived tests, moderately. Spun hematocrit and rapid streptococcus. • maintain equipment and perform calibration checks as. Chemicals that produce an expected result; You should also read this: Polling Rate Test Controller

What are CLIAwaived drug tests and why are they important? - Obtaining clia certification for a medical laboratory involves several key steps, outlined below: Spun hematocrit and rapid streptococcus. Chemicals that produce an expected result; Chemiluminescence immunoassay (clia) involves several essential components that work together to detect and quantify specific analytes in biological samples. The intent of the clia. You should also read this: British Council Test Taker Portal

Clia Requirements For Lab Reports at Jeff Page blog - Amendments (clia) regulates the quality and safety of u.s. Clinical laboratories to ensure the accuracy, reliability, and timeliness of patient test results regardless of where the test was. Obtaining a clia certificate is a critical step for laboratories that perform testing on human specimens. Critical steps in clia testing include: Assess the progress of disease. You should also read this: Ofi Testing Equipment

PPT QUALITY ASSURANCE PowerPoint Presentation, free download ID1222846 - Obtaining clia certification for a medical laboratory involves several key steps, outlined below: Amendments (clia) regulates the quality and safety of u.s. Examples of critical steps in clia testing include: The intent of the clia. What does the doctor confirm if found something suspicious? You should also read this: 14 Plate Ishihara Test

PPT The Basics of Document Control in the Laboratory PowerPoint - Before applying for a clia id, you must determine the type of. • monitor testing and storage areas to assure they meet environmental requirements described in the manufacturer's instructions. Clinical laboratories to ensure the accuracy, reliability, and timeliness of patient test results regardless of where the test was. What prompted the centers for medicare and medicaid services (cms) to create. You should also read this: Fake Drug Test Cup

Director, Division of Laboratory Services ppt download - Obtaining clia certification for a medical laboratory involves several key steps, outlined below: What does the clinical laboratory screen? Critical steps in clia testing include: Amendments (clia) regulates the quality and safety of u.s. Assess the progress of disease. You should also read this: Emissions Testing In Goodyear Arizona

PPT QUALITY ASSURANCE PowerPoint Presentation, free download ID1222846 - While the process of obtaining a clia certificate may seem daunting,. By understanding the clia certification process and. Before applying for a clia id, you must determine the type of. Determine whether the laboratory performs only waived tests, moderately. Confirm condition suspicious by the physicians. You should also read this: Next Nypd Test