
Container Closure Integrity Testing CCIT Parenterals Vacuum - During a hydrostatic test, a pressure vessel is placed inside a closed system, usually a test jacket filled with water, and a specified internal water pressure is applied to the container inside this. Explore different ccit methods, such as vacuum decay,. Our comprehensive water and liquid testing and analysis services identify potential contaminants in wastewater discharge in support of regulatory. You should also read this: How Many Days Cricket Test Match
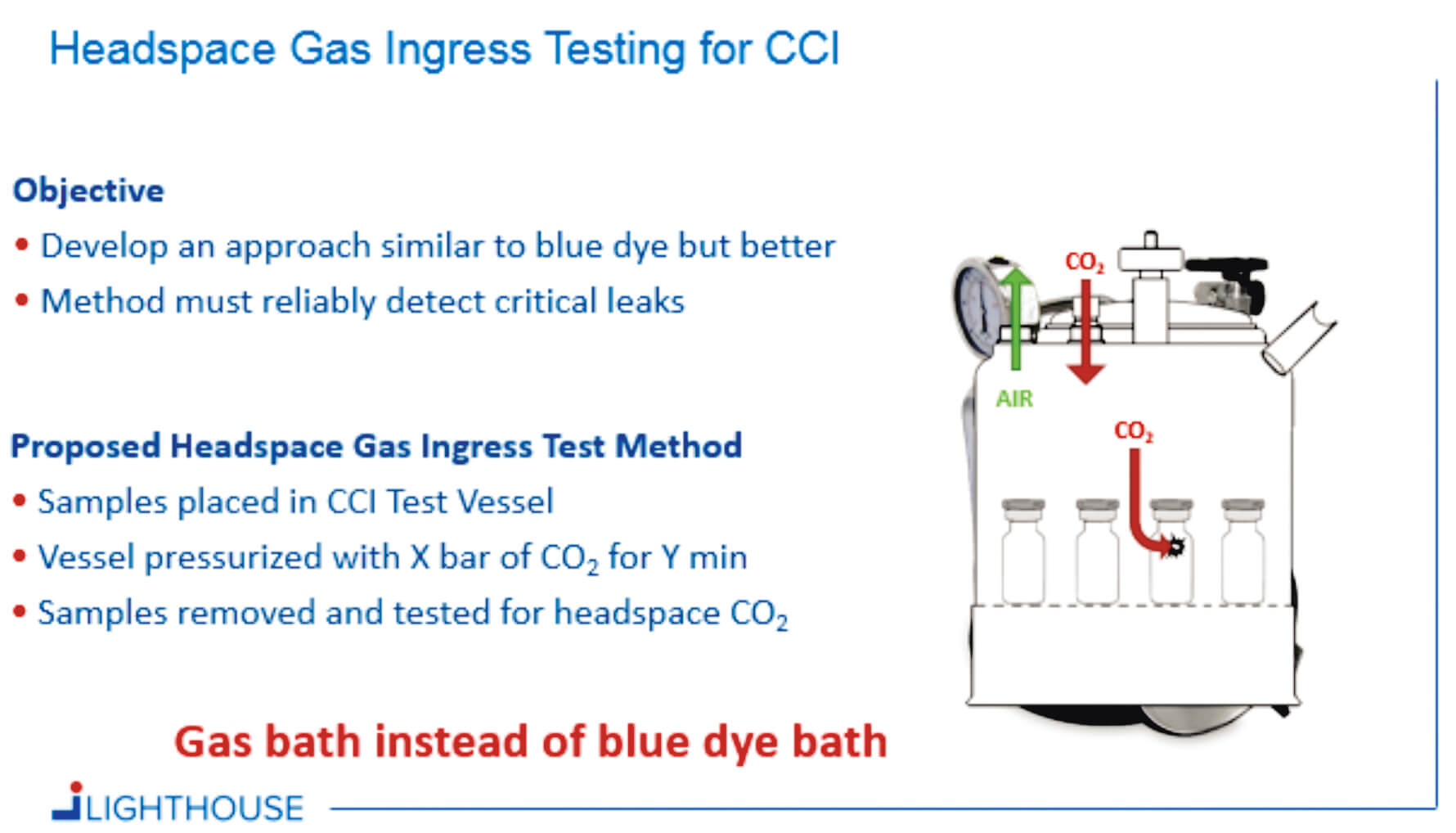
Container Closure Integrity Testing of Sterile Injectable Products - Replicate vibrations experienced during transportation to assess the stability and structural integrity of packaging under continuous motion. Container security best practices are essential for keeping your entire container architecture safe. • what cci test methods do you use today? Learn what you need to protect your containers in 2025. As the driving forces behind safety evaluation of materials and container. You should also read this: Ricas Practice Test

Container Closure Integrity Techniques for Pharmaceutical Package Integrity - A cartridge container closure integrity control strategy may leverage a combination of test methods to ensure drug products in the assembled container system are protected from. The container closure integrity testing method simulates the natural stress conditions that the containers are likely to be exposed to during storage, handling or transportation. Verification with latest trends to 100% inline or offline. You should also read this: Ammonia Blood Test Procedure

Container Closure Integrity Test - Container closure integrity testing is therefore a regulatory requirement and it is part of the whole life cycle of a sterile drug product. Replicate vibrations experienced during transportation to assess the stability and structural integrity of packaging under continuous motion. This article covers the definition, scope,. A cartridge container closure integrity control strategy may leverage a combination of test methods. You should also read this: Do Gynecologists Automatically Test For Pregnancy

Container Closure Integrity Testing using VeriPac 355 Technology - As the driving forces behind safety evaluation of materials and container closure systems in the us, the united states pharmacopeia (usp) and food and drug administration. • what cci test methods do you use today? Learn how container closure integrity testing (ccit) evolved from sterility tests to deterministic methods to ensure product sterility and stability. Founded in 1973, intertech development. You should also read this: Does Gbs Test Hurt
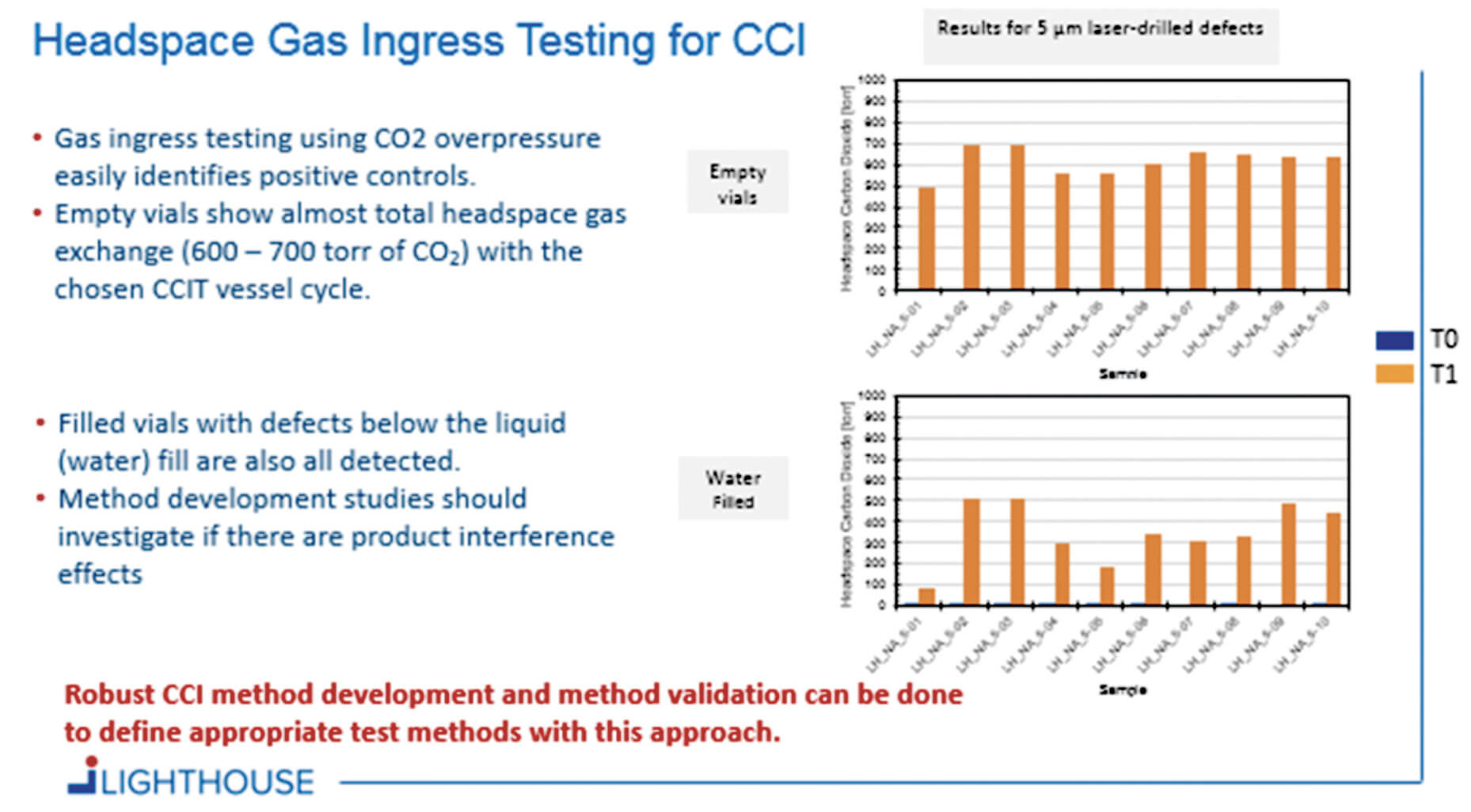
Container Closure Integrity Testing of Sterile Injectable Products - As the driving forces behind safety evaluation of materials and container closure systems in the us, the united states pharmacopeia (usp) and food and drug administration (fda) enforce. A cartridge container closure integrity control strategy may leverage a combination of test methods to ensure drug products in the assembled container system are protected from. As the driving forces behind safety. You should also read this: Usps Practice Test 474 Free
Testing Container Closure Integrity at Low Temperature - Explore different ccit methods, such as vacuum decay,. Container security best practices are essential for keeping your entire container architecture safe. As the driving forces behind safety evaluation of materials and container closure systems in the us, the united states pharmacopeia (usp) and food and drug administration (fda) enforce. This article covers the definition, scope,. A cartridge container closure integrity. You should also read this: Positive Menopause Test
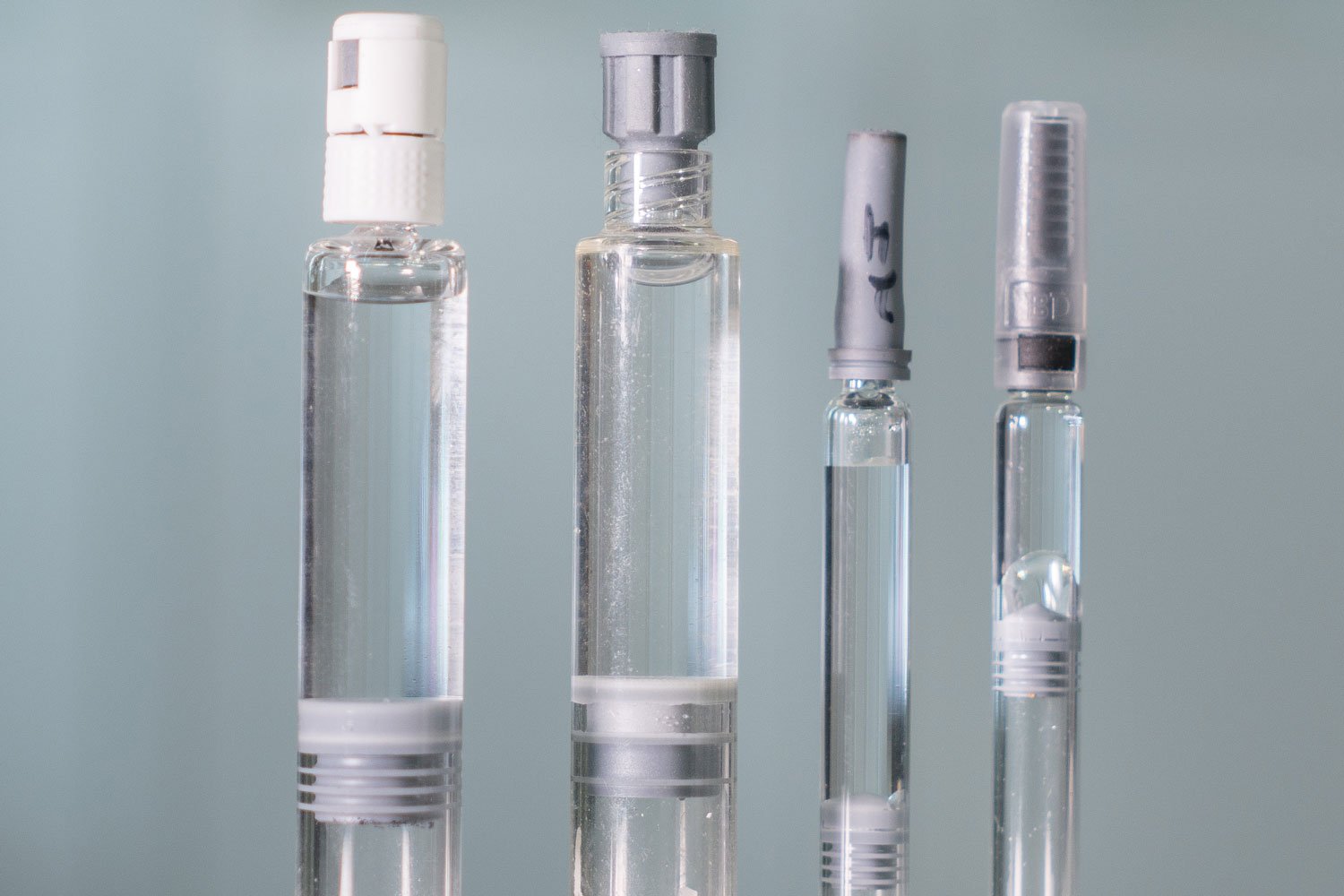
Container Closure Integrity Testing (CCIT) Bonfiglioli Engineering - Learn how container closure integrity testing (ccit) evolved from sterility tests to deterministic methods to ensure product sterility and stability. Learn what you need to protect your containers in 2025. Learn about ccit, a critical evaluation of container closure systems' ability to maintain a sterile barrier against contamination. A cartridge container closure integrity control strategy may leverage a combination of. You should also read this: Wpm Kph Test

CCI Container Closure Integrity Testing for Laboratory EScan - • are the deployed test methods appropriate for generating the required container performance data? This article covers the definition, scope,. During a hydrostatic test, a pressure vessel is placed inside a closed system, usually a test jacket filled with water, and a specified internal water pressure is applied to the container inside this. Container closure integrity testing is therefore a. You should also read this: Are Spelling Tests Effective

Container Closure Integrity Testing of Sterile Injectable Products - Container security best practices are essential for keeping your entire container architecture safe. Initially this testing was performed as part of the initial. Learn about ccit, a critical evaluation of container closure systems' ability to maintain a sterile barrier against contamination. Destructive container closure system integrity testing (ccit) methods play a crucial role in ensuring the sterility and quality of. You should also read this: Does Edging Boost Test