
New FDAapproved blood test could boost screening for deadly colorectal - These tests each have different benefits, limits, and harms (see the table below), and some of them might. Where does it fit in with options like colonoscopy and stool tests? “the way we are approaching blood tests for colon cancer screening is more a complementary option to colonoscopy, or for individuals who may be at higher risk for. The shield. You should also read this: Std Testing Sarasota Fl
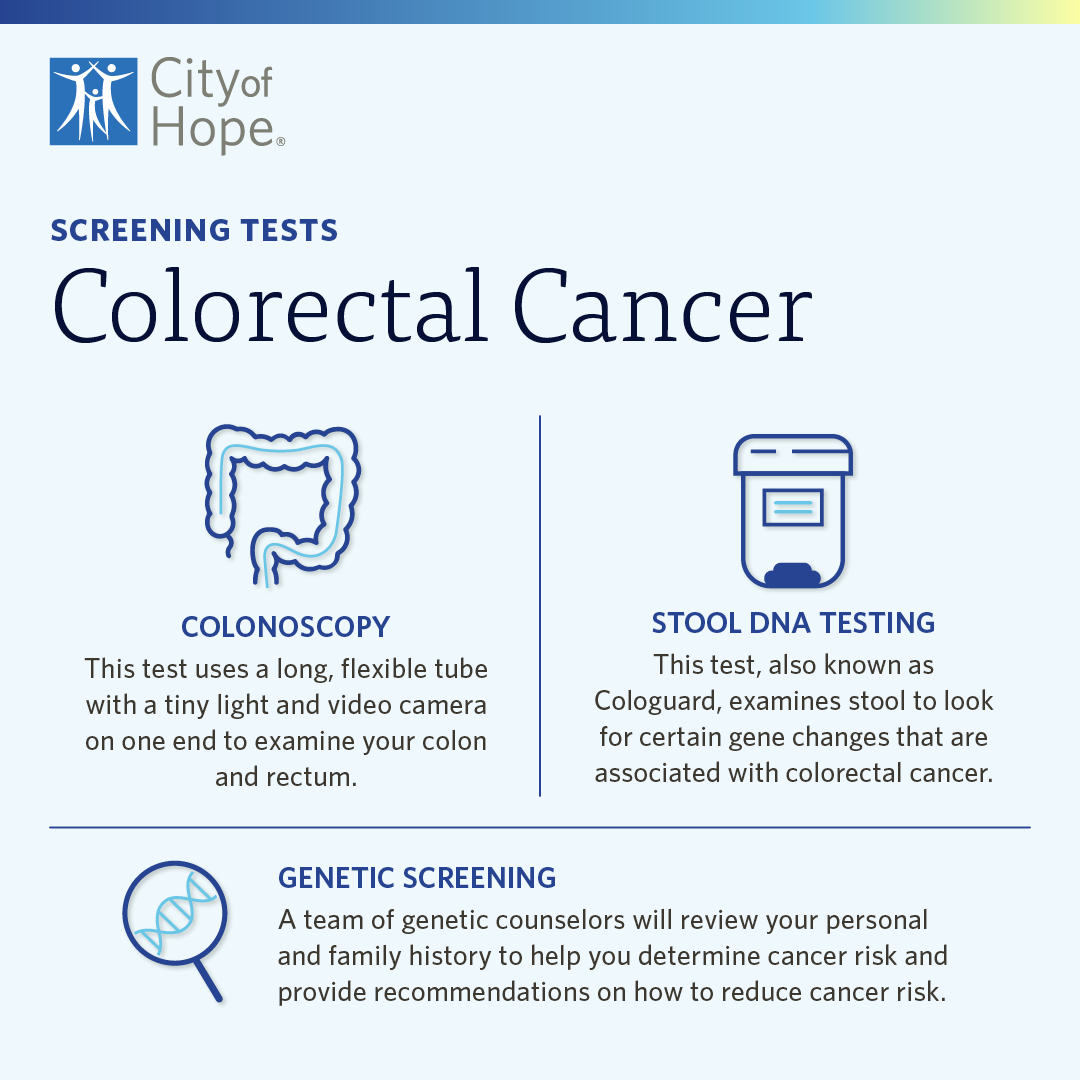
Colorectal cancer screening The test that can save your life City of - They check a sample of your blood for signs that could mean cancer is present. The fda recently approved the test, called shield, for adults age 45 and older who are at average risk for colorectal cancer. The fda approved the test in july 2024 as a primary screening option for colon cancer. A blood test approved in august 2024. You should also read this: Tb Skin Test Icd 10

Colorectal Cancer Screening Options Clinical Gastroenterology and - Fda approved the first blood test for the primary screening of colorectal cancer. What is the new test? The fda approved the test in july 2024 as a primary screening option for colon cancer. This means providers can offer it similarly to other noninvasive screening methods,. There are also blood tests to screen for colon cancer. You should also read this: Waekon Coil Tester

Lab Test For Colorectal Cancer at Savannah Derrington blog - The fda recently approved the test, called shield, for adults age 45 and older who are at average risk for colorectal cancer. These tests check a person's blood for signs of colorectal cancer. Studies have shown that screening. It analyzes plasma dna for certain. Blood tests for colon cancer. You should also read this: Testing For Amoxicillin Allergy
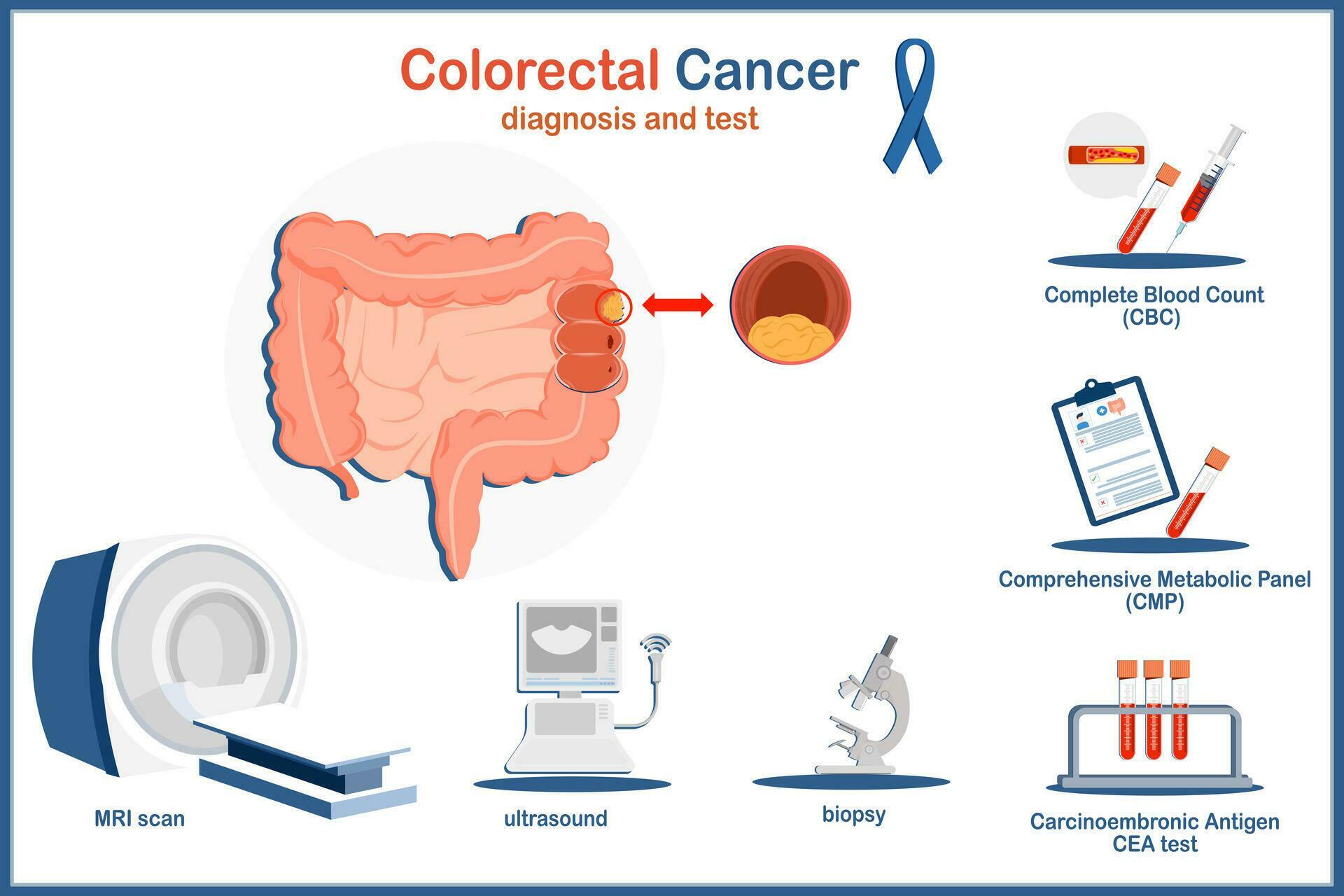
Medical vector illustration concept. Human colon and colon cancer - How can a blood test spot colon cancer? Several test options are available for colorectal cancer screening: Delivered to your doorno prep & no time offpay a billavailable by rx only A blood test approved in august 2024 by the u.s. The fda recently approved the test, called shield, for adults age 45 and older who are at average risk. You should also read this: C Peptide Test Results

Blood Test for Colon Cancer Screening Secures FDA Panel's Blessing - A blood test approved in august 2024 by the u.s. The us food and drug administration has approved the company guardant health’s shield blood test for colorectal cancer screening in adults, 45 and older, who are at average risk for the. Fda approved the first blood test for the primary screening of colorectal cancer. These tests each have different benefits,. You should also read this: How Much Water To Dilute Etg Test
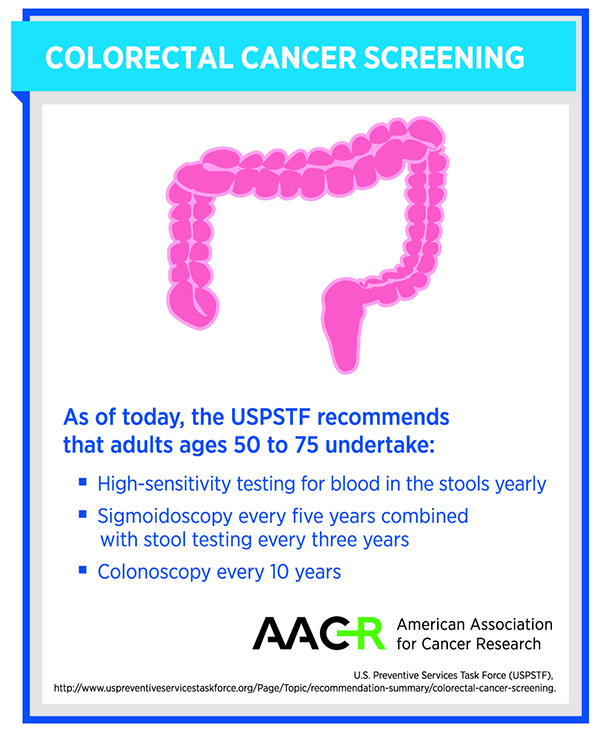
FDA Approves Bloodbased Colorectal Cancer Screening Test American - It analyzes plasma dna for certain. These tests each have different benefits, limits, and harms (see the table below), and some of them might. The shield test detects dna. Markers in the blood can indicate the presence of. There are some differences between these tests to consider (see colorectal cancer screening tests), but the most. You should also read this: New Brunswick.oncourse Connect/test

Colon Cancer Screening Test Ontario at Ron Utecht blog - A blood test approved in august 2024 by the u.s. Where does it fit in with options like colonoscopy and stool tests? The shield test detects dna. Fda approved the first blood test for the primary screening of colorectal cancer. Studies have shown that screening. You should also read this: Mayo Clinic Laboratory Test Catalog
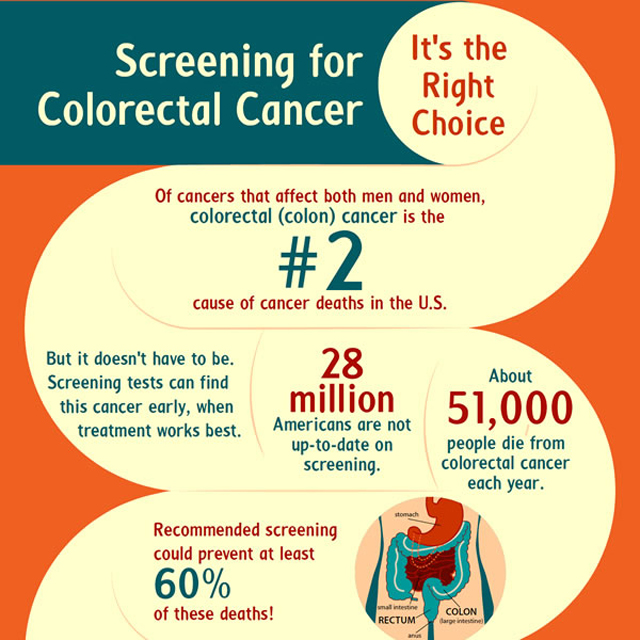
Colon Cancer Test Infographic New Colorectal Cancer Screening - A blood test approved in august 2024 by the u.s. The us food and drug administration has approved the company guardant health’s shield blood test for colorectal cancer screening in adults, 45 and older, who are at average risk for the. Markers in the blood can indicate the presence of. There are also blood tests to screen for colon cancer.. You should also read this: Test For Ma Hopefuls Crossword
COLOTECT, DNA Screening Test for Colorectal Cancer • Innoquest Pathology - The fda approved the test in july 2024 as a primary screening option for colon cancer. The fda recently approved the test, called shield, for adults age 45 and older who are at average risk for colorectal cancer. What is the new test? The shield test detects dna. There are also blood tests to screen for colon cancer. You should also read this: Tandblekning Bäst I Test