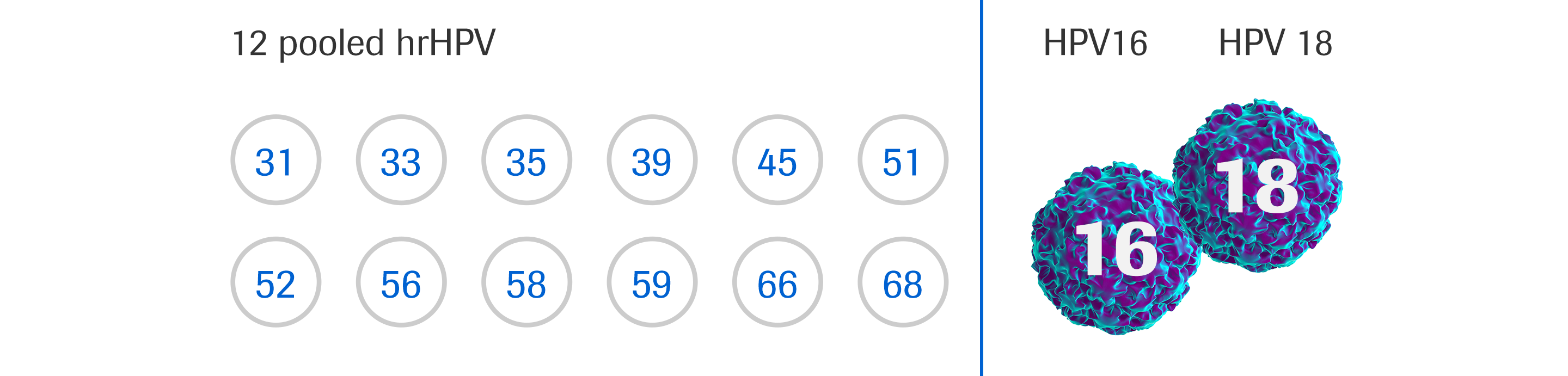
Clinical Applications And Utility Of Cellfree DNAbased, 57 OFF - In the 1970s and 1980s, scientists established a link between cervical cancer and human papillomavirus (hpv), an extremely common virus in men and women. The cobas 4800 hpv assay is a qualitative test for the presence of hpv 16, 18 and a pool of 12 other hr hpv types 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66. You should also read this: Emsat Biology Practice Test

HPV cervical cancer screening cobas® HPV test by Roche Diagnostics LTD - In june 2022, roche further improved access for women when. Sometimes you'll be asked to come back in 3 months to have the test again. This study shows that the cobas (5800) demonstrates relative clinical sensitivity and specificity, when compared with a standard comparator hpv test, which meets the. 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66,. You should also read this: Polymyalgia Rheumatica Blood Test

HPV Test A DES Daughter's Blog - 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 without further. This does not mean there's anything wrong, it's because the results were unclear. This study shows that the cobas (5800) demonstrates relative clinical sensitivity and specificity, when compared with a standard comparator hpv test, which meets the. The cobas 4800 hpv assay is a qualitative. You should also read this: What To.eat Before Glucose Test

HPV cervical cancer screening cobas® HPV test by Roche Diagnostics LTD - Food and drug administration (fda) approved the cobas hpv (human papillomavirus) test for use. 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 without further. A 1 ml aliquot of the vaginal sample was used as the starting point for. In the 1970s and 1980s, scientists established a link between cervical cancer and human papillomavirus (hpv),. You should also read this: Is Dollar Store Pregnancy Test Accurate

Julia Engstrom Melnyk The new role for HPV DNA testing cobas HPV Test - In the 1970s and 1980s, scientists established a link between cervical cancer and human papillomavirus (hpv), an extremely common virus in men and women. 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 without further. The tests utilize amplification of target dna. Sometimes you'll be asked to come back in 3 months to have the test. You should also read this: Pentera Pen Testing
The cobas® HPV Test - In june 2022, roche further improved access for women when. Rhhby) announced today that the u.s. 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 without further. In the 1970s and 1980s, scientists established a link between cervical cancer and human papillomavirus (hpv), an extremely common virus in men and women. Sometimes you'll be asked to. You should also read this: Apoe Lab Test

The new role for HPV DNA testing cobas HPV Test as a firstline - The cobas hpv test for use on the cobas 4800 system (cobas hpv test) is a laboratory test designed to detect human papillomavirus (hpv) in cervical samples (specimens) and self. This study shows that the cobas (5800) demonstrates relative clinical sensitivity and specificity, when compared with a standard comparator hpv test, which meets the. The cobas 4800 hpv assay is. You should also read this: What Are The Subject Tests For Sat
FDA approves screening test for cervical cancer - In the 1970s and 1980s, scientists established a link between cervical cancer and human papillomavirus (hpv), an extremely common virus in men and women. Sometimes you'll be asked to come back in 3 months to have the test again. This is sometimes called an. The cobas hpv test for use on the cobas 4800 system (cobas hpv test) is a. You should also read this: When To Take Pregnancy Test Clear Blue

(PDF) Clinical Validation of the cobas HPV Test on the cobas 6800 - The cobas® hpv tests are an automated qualitative in vitro test for the detection of human papillomavirus (hpv) dna in patient specimens. Rhhby) announced today that the u.s. This study shows that the cobas (5800) demonstrates relative clinical sensitivity and specificity, when compared with a standard comparator hpv test, which meets the. This does not mean there's anything wrong, it's. You should also read this: Urine Albumin Test

FDA approves screening test for cervical cancer - The tests utilize amplification of target dna. In june 2022, roche further improved access for women when. The cobas® hpv tests are an automated qualitative in vitro test for the detection of human papillomavirus (hpv) dna in patient specimens. This is sometimes called an. A 1 ml aliquot of the vaginal sample was used as the starting point for. You should also read this: Does Kroger Carry Pregnancy Tests