
PPT QUALITY ASSURANCE PowerPoint Presentation, free download ID1222846 - And ( b ) meet one of the following requirements: ( a ) each individual. The onus for following clia regulations and ensuring that test results are accurate and reliable falls on the clinical testing laboratory, regardless of whether it uses an ldt or. 1 the license to practice medicine must be. List of clia requirements + responsibilities for testing. You should also read this: Accurate Hair Porosity Test

PPT QUALITY ASSURANCE PowerPoint Presentation, free download ID1409864 - 1 the license to practice medicine must be. Documented competency assessment is required for individuals fulfilling the following personnel responsibilities outlined in subpart m of the clia regulations: Clia certification is required for all medical laboratories that perform testing on human specimens in the united states. The testing personnel are responsible for specimen processing, test performance and for reporting test. You should also read this: Brain Check Test Free
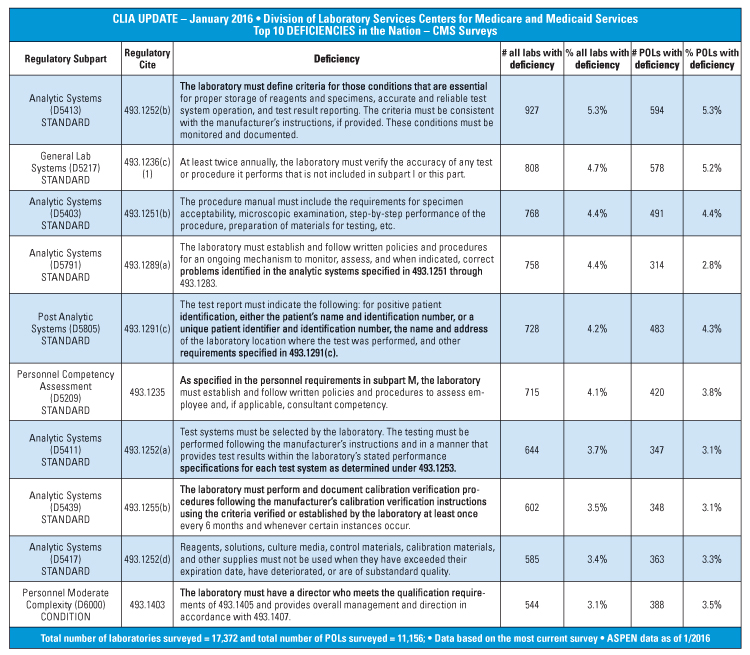
CLIA and regulatory readiness How can your lab always be ready - Federal regulations were created to mandate the minimum qualifications of laboratory testing personnel. The testing personnel are responsible for specimen processing, test performance, and for reporting test results. The testing personnel are responsible for specimen processing, test performance and for reporting test results. December 2024—the new edition of the cap accreditation program checklists will contain revised requirements for the qualification. You should also read this: Pure Encapsulations Third Party Testing

PPT Complying With CLIA Competency Requirements PowerPoint - The use of a psv report as evidence of meeting the clinical laboratory improvement amendments of 1988 (clia) personnel qualifications is optional for laboratories. December 2024—the new edition of the cap accreditation program checklists will contain revised requirements for the qualification of laboratory directors, technical and general. The testing personnel are responsible for specimen processing, test performance and for reporting. You should also read this: How To Test A Aa Battery Without A Tester

Director, Division of Laboratory Services ppt download - The testing personnel are responsible for specimen processing, test performance and for reporting test results. December 2024—the new edition of the cap accreditation program checklists will contain revised requirements for the qualification of laboratory directors, technical and general. Clia certification is required for all medical laboratories that perform testing on human specimens in the united states. The requirements for obtaining. You should also read this: Can I Have A False Positive Covid Test

Director, Division of Laboratory Services ppt download - December 2024—the new edition of the cap accreditation program checklists will contain revised requirements for the qualification of laboratory directors, technical and general. Each individual performing high complexity testing must— (a) possess a current license issued by the state in which the laboratory is. Clia certification is required for all medical laboratories that perform testing on human specimens in the. You should also read this: Heathkit Tube Tester

PPT CLIA PowerPoint Presentation, free download ID573645 - And ( b ) meet one of the following requirements: Each individual performing high complexity testing must— ( a ) possess a current license issued by the state in which the laboratory is located, if such licensing is required; The changes to the personnel section (subpart m) of the clia regulations, is available for review in the federal register; Changes. You should also read this: Lung Test Online Timer

PPT CLIA Waived Testing for Physician Office Labs PowerPoint - ( a ) each individual. Each individual performing high complexity testing must— (a) possess a current license issued by the state in which the laboratory is. High complexity and moderate complexity laboratories. Changes to subpart m will take effect on december 28, 2024. Clia certification is required for all medical laboratories that perform testing on human specimens in the united. You should also read this: Asbestos Air Testing Kit Home Depot

Clia Requirements For Lab Reports at Jeff Page blog - The use of a psv report as evidence of meeting the clinical laboratory improvement amendments of 1988 (clia) personnel qualifications is optional for laboratories. List of clia requirements + responsibilities for testing personnel in the u.s. Cms and cdc are finalizing the proposed updates to the clia regulations. December 2024—the new edition of the cap accreditation program checklists will contain. You should also read this: Hydrogen Breath Test Quest

Safety and Regulatory Guidelines In the Medical Laboratory ppt download - Cms and cdc are finalizing the proposed updates to the clia regulations. List of clia requirements + responsibilities for testing personnel in the u.s. The changes to the personnel section (subpart m) of the clia regulations, is available for review in the federal register; December 2024—the new edition of the cap accreditation program checklists will contain revised requirements for the. You should also read this: Atb Test Practice