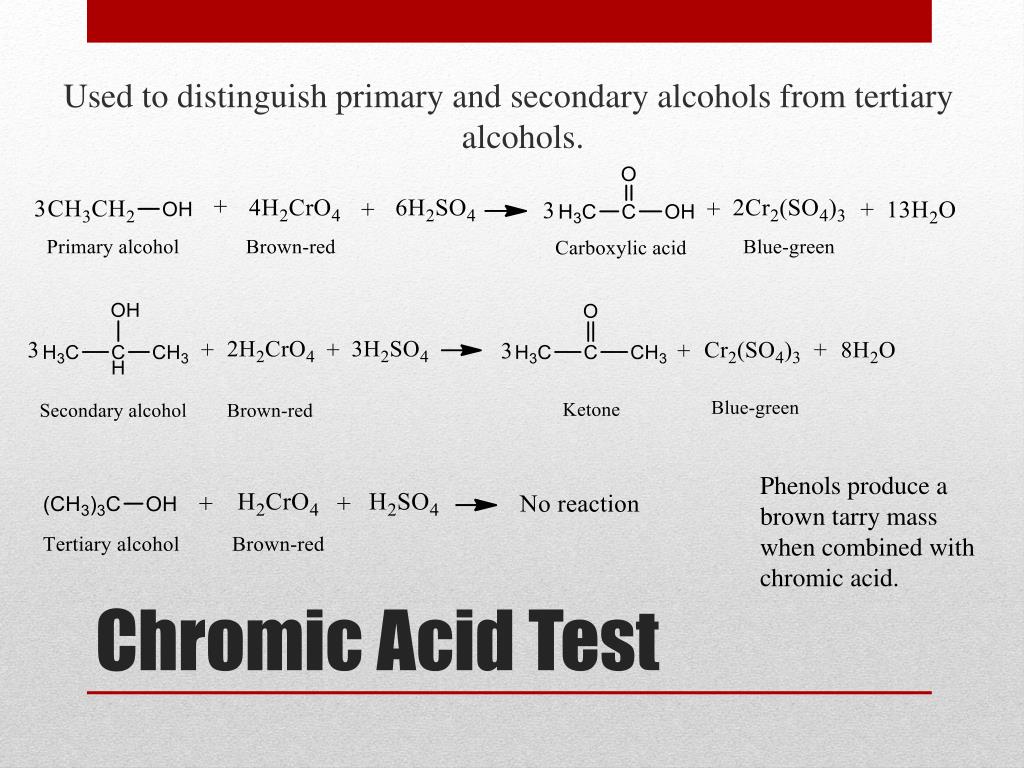
PPT Classification and Identification of Alcohols and Phenols - Some of the primary and secondary alcohols also give this test but. A chromic acid test is used to detect the presence of alcohols, specifically primary and secondary alcohols. Chromic acid oxidation this test distinguishes primary and secondary alcohols from tertiary. The functional groups that will be studied in this experiment are carboxylic acid, amines aldehyde, ketone, alcohols and alkenes.. You should also read this: Strep Test Abnormal

Solved Chromic Acid Test Oxidation of Alcohols Alcohol - Chromic acid oxidation this test distinguishes primary and secondary alcohols from tertiary. Aldehydes react with chromic acid gives a green to blue precipitate. Chromic acid (h₂cro₄) is a strong oxidizing agent that reacts with primary and secondary. Ketones do not react with chromic acid. The chromic acid test is used to detect the presence of secondary alcohols. You should also read this: 2 Hour Glucose Tolerance Test Postpartum Normal Range
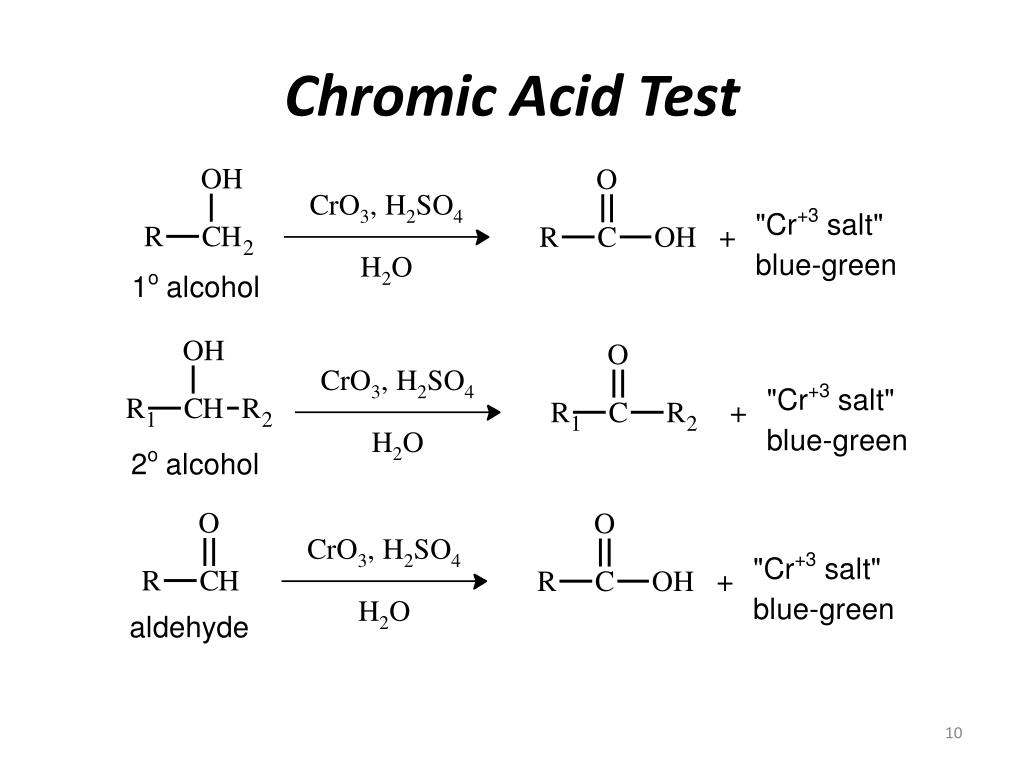
PPT Identification of an Unknown OxygenContaining Compound - In this experiment, we will be using four of these tests to identify an unknown organic compound. A chemical test is typically a fast reaction performed in a test tube that gives a dramatic visual clue (a color change, precipitate, or gas formation) as evidence for a chemical reaction. It is also true that other functional groups, primary and secondary. You should also read this: Whirlpool Dishwasher Drain Pump Test
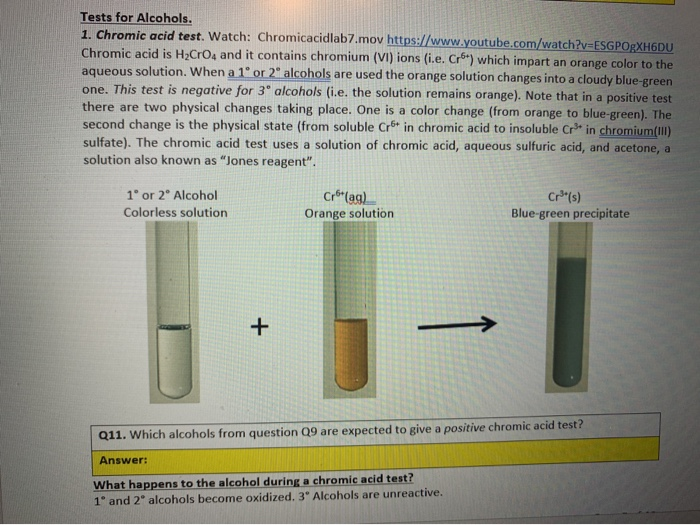
Alcohol Chromic Acid Test - The chromic acid test is used to detect aldehydes and alcohols. Ketones do not react with chromic acid. Ketones do not react with chromic acid. Aldehydes react with chromic acid gives a green to blue precipitate. Add a few drops of chromic acid. You should also read this: Allied Universal Drug Test Reddit
Alcohol Chromic Acid Test Chromic Acid Test for Aldehydes & Alcohols - The chromic acid test is used to measure alcohols and aldehydes. It involves adding chromic acid to the compound and observing a color change. The chromic acid test is used to detect aldehydes and alcohols. Consequently, chromic acid can distinguish between aldehydes and ketones. Some of the primary and secondary alcohols also give this test but. You should also read this: A1 Smog Test
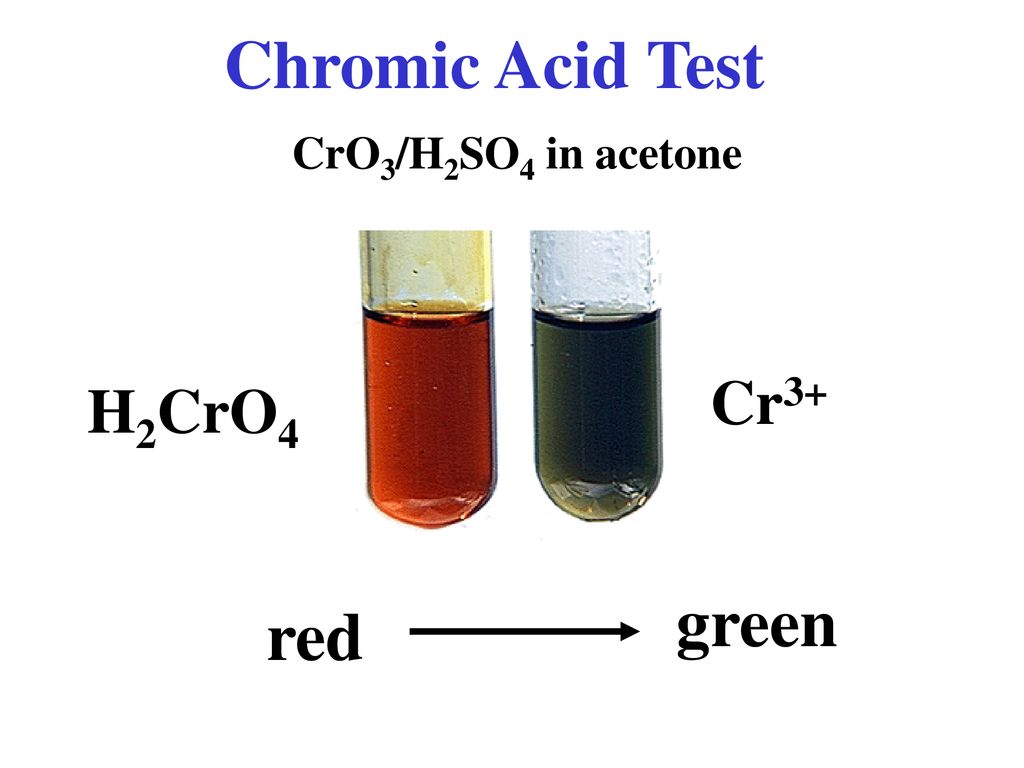
Unknown 1 Type of Compound Alcohol Aldehyde Ketone. ppt download - Consequently, chromic acid can distinguish between aldehydes and ketones. Ketones do not react with chromic acid. It is also true that other functional groups, primary and secondary alcohols for example, can be oxidized by. Some of the primary and secondary alcohols also give this test but. You will learn chemical tests that will allow you to distinguish. You should also read this: Google Phone App Testing Iphone-style Incoming Call Screen
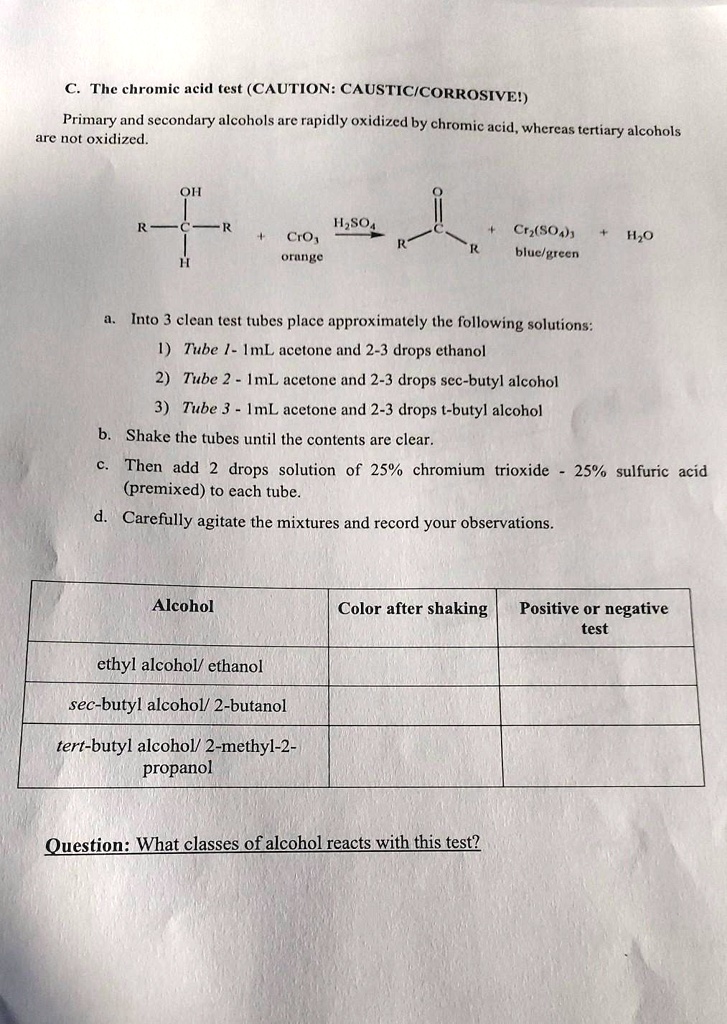
SOLVED The chromic acid test (CAUTION CAUSTIC CORROSIVE) Primary and - Aldehydes react with chromic acid gives a green to blue precipitate. Learn how the test is used by law enforcement, the properties of the jones reactant. Chromic acid oxidation this test distinguishes primary and secondary alcohols from tertiary. Indications of a positive test: The tests being utilized include the following: You should also read this: Staph Catalase Test

Chromic Acid Test for Aldehydes & Alcohols Mechanism Lesson - Consequently, chromic acid can distinguish between aldehydes and ketones. Chromic acid will oxidize a primary alcohol first to an aldehyde and then to a. Aldehydes react with chromic acid gives a green to blue precipitate. Add a few drops of chromic acid. Chromic acid (h₂cro₄) is a strong oxidizing agent that reacts with primary and secondary. You should also read this: Plum Personality Test

Alkanes, alkenes, alcohols, aldehydes and ketones ppt download - The tests being utilized include the following: A chemical test is typically a fast reaction performed in a test tube that gives a dramatic visual clue (a color change, precipitate, or gas formation) as evidence for a chemical reaction. Learn how the test is used by law enforcement, the properties of the jones reactant. You will learn chemical tests that. You should also read this: Autonomic Response Testing Ireland

Unknown 1 Type of Compound Alcohol Aldehyde Ketone. ppt download - Dissolve 10 mg of a solid (or 1 drop of a liquid) unknown in reagent grade acetone in a clean, dry test tube. The chromic acid test is used to distinguish between primary, secondary, and tertiary alcohols. Ketones do not react with chromic acid. Primary and secondary alcohols can be oxidized by chromic acid to form carboxylic acid, while a. You should also read this: Megger Tester 1000v