
Oxidation by Chromic Acid Chemistry LibreTexts - It is also true that other functional groups, primary and secondary alcohols for example, can be oxidized by. Tertiary alcohols give a negative result with this test (figure. Learn how the test is used by law enforcement, the properties of the jones reactant. Oxidation of alcohols by chromic acid is much greater in an acidic solution. Chromic acid (h₂cro₄) oxidizes. You should also read this: Does Walgreens Have Drug Tests
Oxidation by Chromic Acid Chemistry LibreTexts - Three drops of the compound to be tested are mixed with 5 drops of acetone and 5 drops of chromic acid solution (an orange solution). Learn how the test is used by law enforcement, the properties of the jones reactant. Chromic acid oxidation this test distinguishes primary and secondary alcohols from tertiary. Oxidation of alcohols by chromic acid is much. You should also read this: What Hypothesis Have The Grants Been Testing
Oxidation by Chromic Acid Chemistry LibreTexts - The chromic acid test is used to measure alcohols and aldehydes. How to perform the test: This test distinguishes between primary and secondary alcohols (and aldehydes), which are oxidized by chromic acid (h ₂ cro ₄), and tertiary. Chromic acid will oxidize a primary alcohol first to an aldehyde and then to a. Consequently, chromic acid can distinguish between aldehydes. You should also read this: Sentara Test Menu

Jones Reagent Organic Chemistry at Orville Turner blog - The chromic acid test uses the jones reactant to oxidize aldehydes and alcohols and reduce the chromic acid, resulting in a color change. Chromic acid oxidation this test distinguishes primary and secondary alcohols from tertiary. It is able to identify aldehydes, primary alcohol, and. Chromic acid will oxidize a primary alcohol first to an aldehyde and then to a. Mechanism. You should also read this: Ap Psych Sensation And Perception Practice Test
Oxidation by Chromic Acid Chemistry LibreTexts - Learn how the test is used by law enforcement, the properties of the jones reactant. The reaction results in a color change from. This test distinguishes between primary and secondary alcohols (and aldehydes), which are oxidized by chromic acid (h ₂ cro ₄), and tertiary. The jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric. You should also read this: Icc Practice Test

Alcohol Oxidation Mechanisms and Practice Problems Chemistry Steps - Chromic acid test the chromic acid test, also known as the jones test, is based in the chromic acid oxidation of the alcohols and aldehydes to the corresponding carbonyl compound. How to perform the test: Consequently, chromic acid can distinguish between aldehydes and ketones. Chromic acid (h₂cro₄) oxidizes alcohols and. A solution of \(\ce{cro_3}\) in \(\ce{h_2so_4}\) is a test for. You should also read this: Wisconsin Emission Testing Sites
Jones oxidation - Learn how the test is used by law enforcement, the properties of the jones reactant. It uses chromic acid, a strong oxidizing agent, to oxidize aldehydes and alcohols to carboxylic acids, reducing the chromic. It is also true that other functional groups, primary and secondary alcohols for example, can be oxidized by. How to perform the test: Chromic acid will. You should also read this: Free Std Testing Columbia Sc
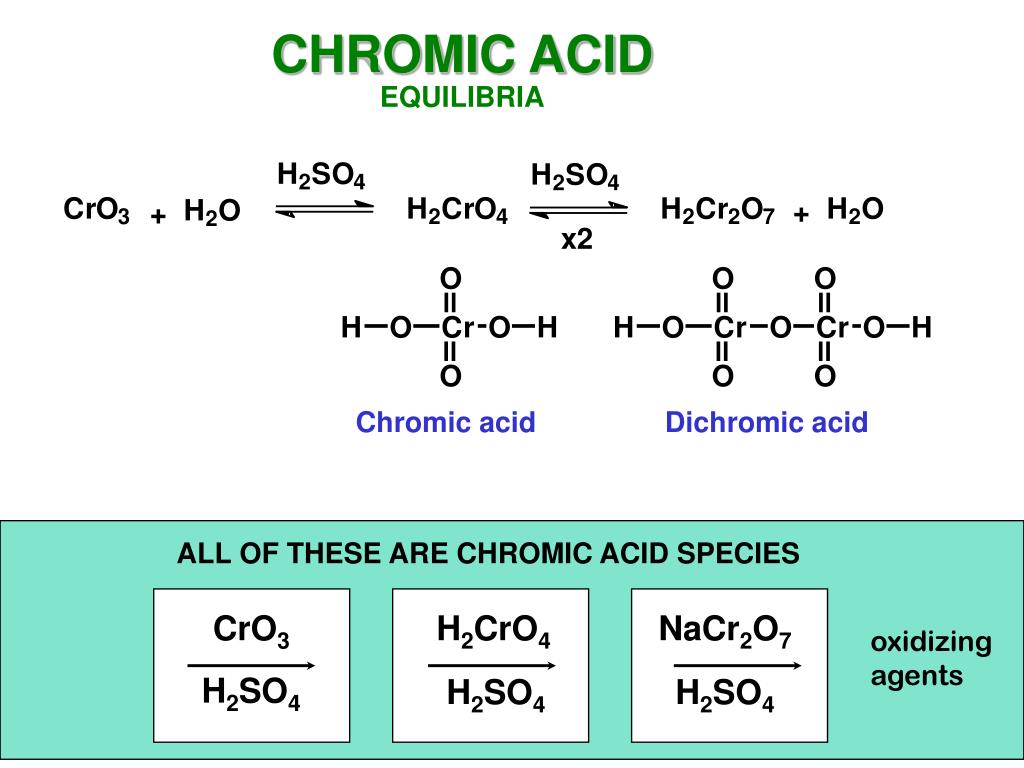
PPT OXIDATIONS OF ALCOHOLS PowerPoint Presentation, free download - Chromic acid (h₂cro₄) is a strong oxidizing agent that reacts with primary and secondary alcohols to form aldehydes and ketones, respectively. Consequently, chromic acid can distinguish between aldehydes and ketones. This test distinguishes between primary and secondary alcohols (and aldehydes), which are oxidized by chromic acid (h ₂ cro ₄), and tertiary. The chromic acid test is used to measure. You should also read this: Livongo Test Strips Where To Buy
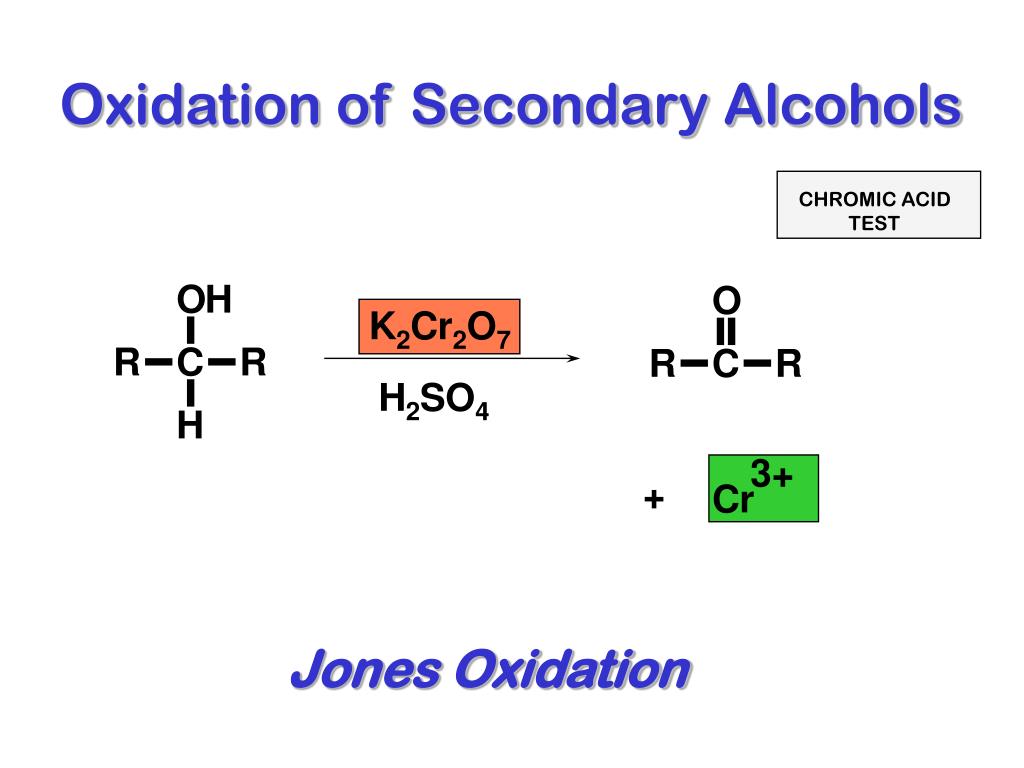
PPT OXIDATIONS OF ALCOHOLS PowerPoint Presentation, free download - It is able to identify aldehydes, primary alcohol, and. Chromic acid will oxidize a primary alcohol first to an aldehyde and then to a. Consequently, chromic acid can distinguish between aldehydes and ketones. It uses chromic acid, a strong oxidizing agent, to oxidize aldehydes and alcohols to carboxylic acids, reducing the chromic. Oxidation of alcohols by chromic acid is much. You should also read this: Emissions Testing Highlands Ranch Co
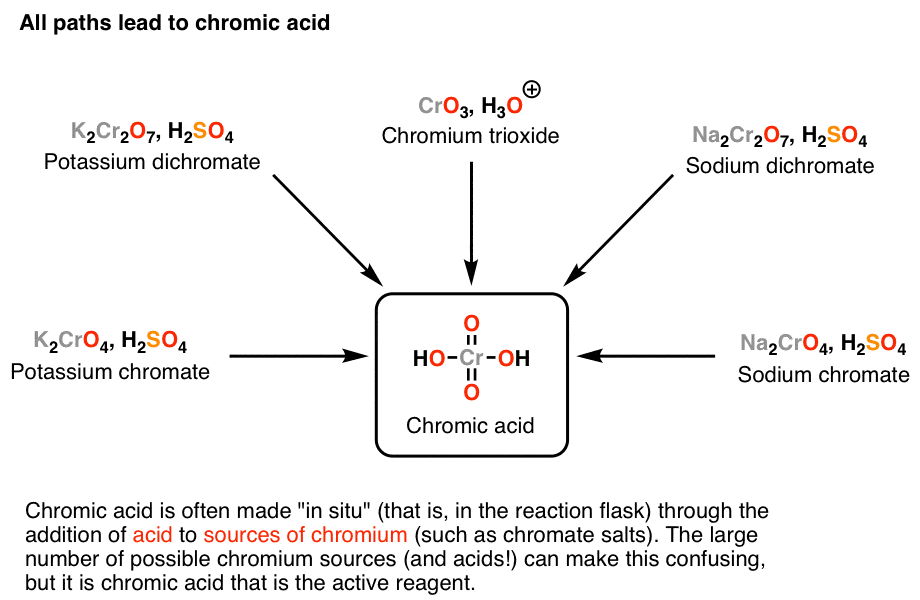
10.6.2. Strong Oxidizing Agents Chemistry LibreTexts - Chromic acid (h₂cro₄) is a strong oxidizing agent that reacts with primary and secondary alcohols to form aldehydes and ketones, respectively. Chromic acid oxidation this test distinguishes primary and secondary alcohols from tertiary. The jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms chromic acid in situ. The chromic acid test is. You should also read this: Can I Drink Coffee Before My 1 Hour Glucose Test