![ICD10CM Diagnosis Code R97.0 Elevated carcinoembryonic antigen [CEA] ICD10CM Diagnosis Code R97.0 Elevated carcinoembryonic antigen [CEA]](https://icdlist.com/assets/icd-10/r/R970.jpg)
ICD10CM Diagnosis Code R97.0 Elevated carcinoembryonic antigen [CEA] - Results obtained with different assay methods or kits cannot be used interchangeably. This assay is intended for the in vitro quantitative determination of carcinoembryonic antigen in human serum and plasma. Liver cirrhosis, chronic hepatitis, pancreatitis, ulcerative. Carcinoembryonic antigen (cea) is a protein polysaccharide found in some carcinomas. The cpt codes provided in this document are based on ama guidelines and. You should also read this: Lachman Test Negative

Printable Labcorp Tube Color Chart - This test may exhibit interference when sample is collected from a person who is consuming a supplement. Carcinoembryonic antigen (cea) is a protein polysaccharide found in some carcinomas. 82378 details this assay is intended for the in vitro quantitative determination of carcinoembryonic antigen in human serum and plasma. Peritoneal, abdominal, ascites, paracentesis fluid (peritoneal washings are not acce ptable) container/tube:. You should also read this: Editable Spelling Test Template

Will I pass?? Labcorp 10 panel drugtesthelp - The cpt codes provided in this document are based on ama guidelines and are for informational purposes only. Nocpt codes for this test: 1 the elecsys cea assay is further indicated for serial. This assay is intended for the in vitro quantitative determination of carcinoembryonic antigen in human serum and plasma. For a complete test description, see carcinoembryonic antigen (cea). You should also read this: Tb Test San Antonio

Labcorp Provides HbA1c Testing With AtHome Collection Kit MPR - The roche cea electrochemiluminescent immunoassay is used. Liver cirrhosis, chronic hepatitis, pancreatitis, ulcerative. Direct access testing with or without insurance. The cpt codes provided in this document are based on ama guidelines and are for informational purposes only. Results obtained with different assay methods or kits cannot be used interchangeably. You should also read this: Post Test: The Contemporary World

Common vacutainers for blood samples and color codes. Image Source - This assay is intended for the in vitro quantitative determination of carcinoembryonic antigen in human serum and plasma. Cea measures the levels of a protein associated with certain cancers, particularly in the colon and rectum. The main indication for cea. Cea (carcinoembryonic antigen) blood test (labcorp) this test measures the level of carcinoembryonic antigen (cea) in the blood. Peritoneal, abdominal,. You should also read this: Monospot Test Kit

Carcinoembryonic Antigen (CEA) Rapid Test - Cpt coding is the sole responsibility of the billing party. 1 microtainer filled to top line: 82378 details this assay is intended for the in vitro quantitative determination of carcinoembryonic antigen in human serum and plasma. Cea is a protein which is often elevated in. The cpt codes provided in this document are based on ama guidelines and are for. You should also read this: S Pyogenes Catalase Test

Lipid Labcorp Test Code at davidddahlkeo blog - The roche cea electrochemiluminescent immunoassay is used. Liver cirrhosis, chronic hepatitis, pancreatitis, ulcerative. 1 the elecsys cea assay is further indicated for serial measurement of cea to aid in the management of cancer patients. This assay is intended for the in vitro quantitative determination of carcinoembryonic antigen in human serum and plasma. The cea assay value, regardless of. You should also read this: Does Trazodone Show On Drug Test
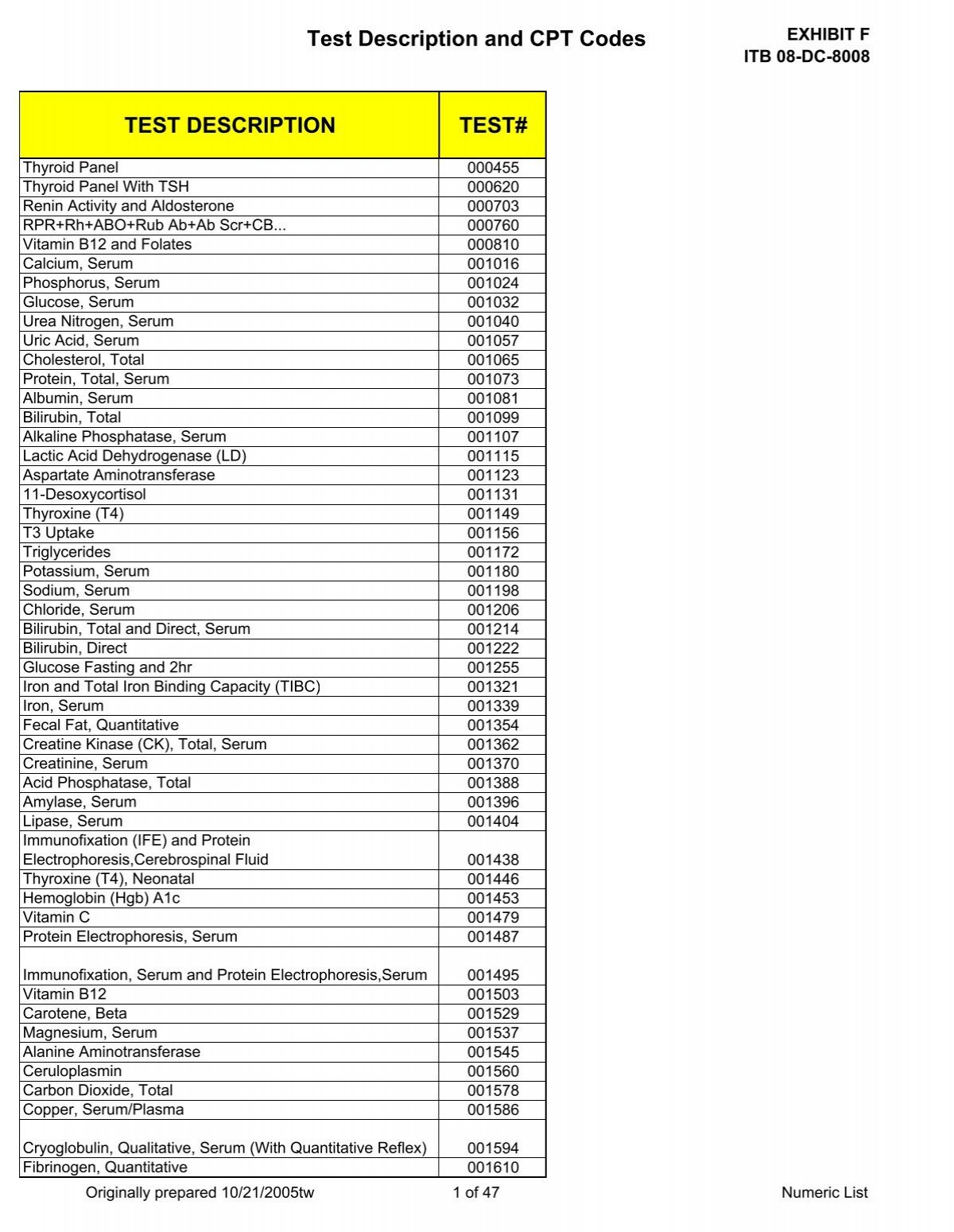
Labcorp Test Code For Urinalysis With Reflex To Culture at Joseph Kogan - Cpt coding is the sole responsibility of the billing party. Labcorp oncology offers a comprehensive test menu to support you in the diagnosis and treatment of patients with colorectal cancer throughout their continuum of care,. 1 microtainer filled to top line: Cea measures the levels of a protein associated with certain cancers, particularly in the colon and rectum. It is. You should also read this: Full Panel Std Test Planned Parenthood

Images Phlebotomy study, Phlebotomy, Order of draw - The main indication for cea. Cea is a protein which is often elevated in. It is effective as a biochemical marker for monitoring the response of certain malignancies to. This assay is intended for the in vitro quantitative determination of carcinoembryonic antigen in human serum and plasma. 1 the elecsys cea assay is further indicated for serial. You should also read this: Does Us Xpress Do Hair Follicle Test
Herpes Culture Labcorp Test Code at Linda Dupree blog - Elevated cea levels are seen in breast, colon, lung, pancreas, rectum, and stomach cancers. This assay is intended for the in vitro quantitative determination of carcinoembryonic antigen in human serum and plasma. 82378 details this assay is intended for the in vitro quantitative determination of carcinoembryonic antigen in human serum and plasma. Clearly indicate clinical reason for testing provide the. You should also read this: Is The Dollar Tree Pregnancy Test Accurate