
Container Closure Integrity Tests CCIT PDF Growth Medium - But with multiple methods to choose. With reproducible and predictable results, deterministic test methods represent the most reliable form of container closure integrity testing. It involves evaluating the integrity of. Container closure integrity (cci) testing supports the sterility and quality of pharmaceutical products, meeting regulatory requirements and safeguarding public health. Container closure integrity testing is done to evaluate the strength. You should also read this: Cvs Pregnancy Test Aisle
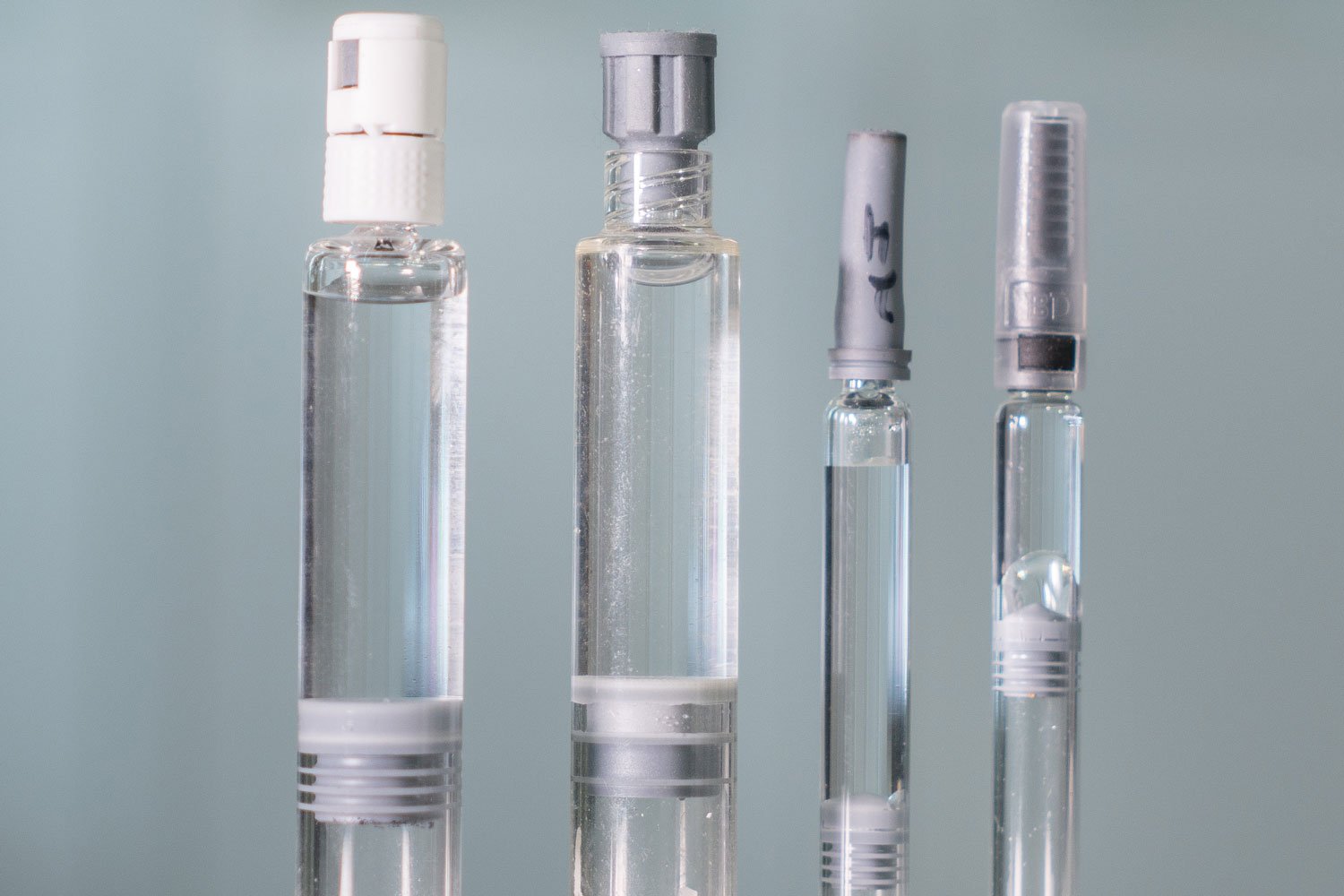
Container Closure Integrity Testing (CCIT) Bonfiglioli Engineering - Container closure integrity testing (ccit) involves evaluating packaging systems to determine their ability to protect the stability and sterility of pharmaceutical products. It involves evaluating the integrity of. A cartridge container closure integrity control strategy may leverage a combination of test methods to ensure drug products in the assembled container system are protected from. The container closure integrity testing method. You should also read this: Asbestos Testing Dallas

Container Closure Integrity Testing (CCIT) (Analysis Make Pressure - Container closure integrity testing (ccit) involves evaluating packaging systems to determine their ability to protect the stability and sterility of pharmaceutical products. Container closure integrity testing (ccit) is an assay that evaluates the adequacy of container closure systems to maintain a sterile barrier against potential contaminants. This critical quality assurance process prevents. Container closure integrity testing is done to evaluate. You should also read this: Ccf Test Directory
Container Closure Integrity Testing (CCIT) safety in numbers - Container closure integrity testing is done to evaluate the strength and integrity of a container closure system. Container closure integrity testing (ccit) is an assay that evaluates the adequacy of container closure systems to maintain a sterile barrier against potential contaminants. Container closure integrity (cci) testing supports the sterility and quality of pharmaceutical products, meeting regulatory requirements and safeguarding public. You should also read this: What Happens If You Get Caught Cheating On Dmv Test
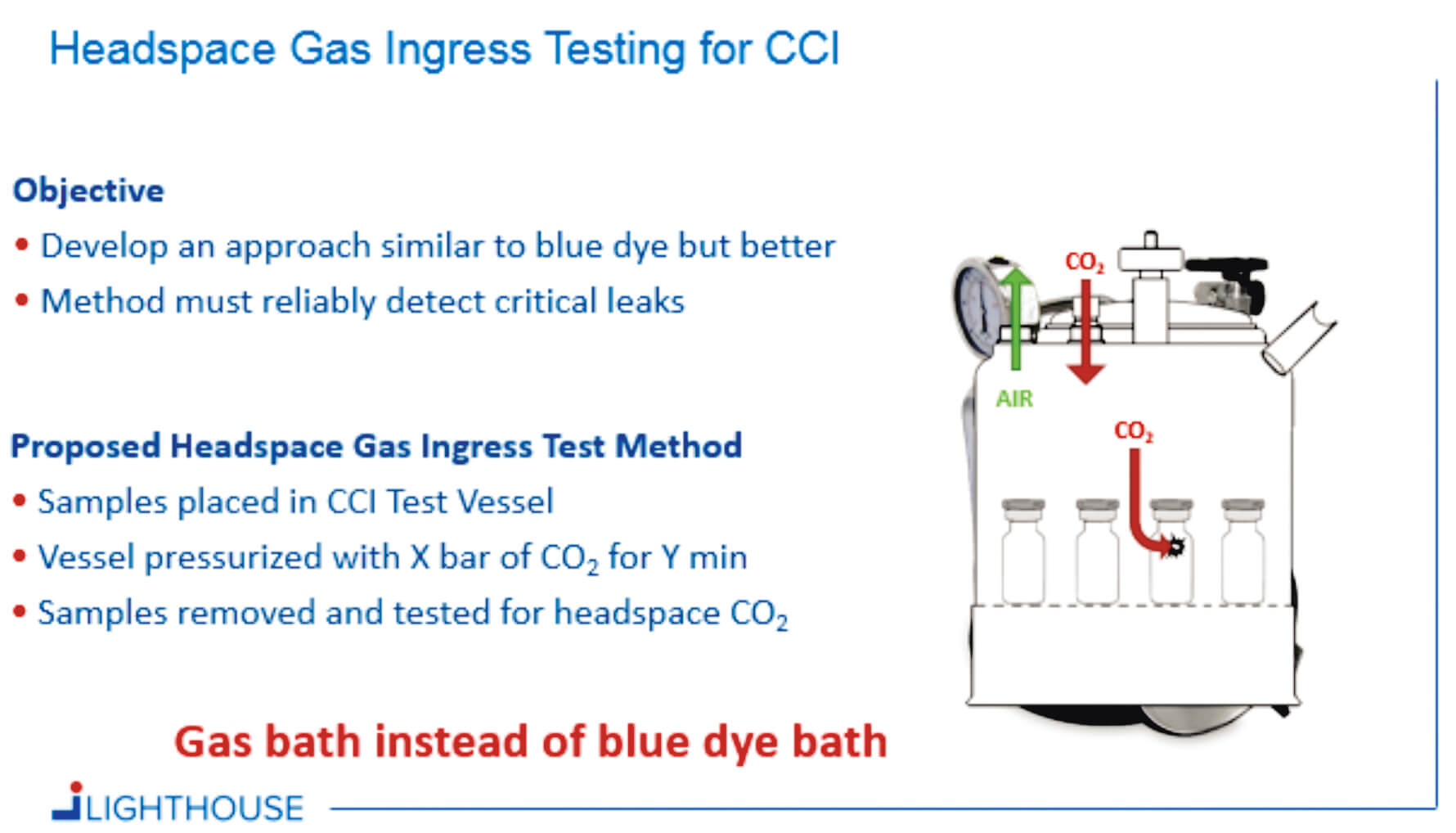
Container Closure Integrity Testing of Sterile Injectable Products - Container closure integrity testing (ccit) is a crucial process that ensures the packaging of sterile vials maintains a sterile barrier against potential contaminants. Ccit serves as a reliable method to verify that packaging. Container closure integrity testing evaluates how effectively packaging systems maintain sterile barriers against potential contaminants. Container closure integrity testing is done to evaluate the strength and integrity. You should also read this: Ohio Drivers Maneuverability Test

Container Closure Integrity Testing (CCIT) validation the advantages - Container closure integrity testing is done to evaluate the strength and integrity of a container closure system. This is particularly important for. Container closure integrity testing (ccit) is a crucial quality control measure for pharmaceutical and medical product packaging. This critical quality assurance process prevents. Container closure integrity (cci) testing supports the sterility and quality of pharmaceutical products, meeting regulatory. You should also read this: Newaygo Drivers Test

Container Closure Integrity Testing CCIT YouTube - Container closure integrity testing (ccit) is a crucial process that ensures the packaging of sterile vials maintains a sterile barrier against potential contaminants. A container closure integrity test assesses the integrity of container closures to prevent content leakage and the ingress of moisture, air, or contaminants. A cartridge container closure integrity control strategy may leverage a combination of test methods. You should also read this: Best Movie Scenes To Test Surround Sound

Container Closure Integrity Testing (CCIT) An Ideal Solution for the - Container closure integrity testing (ccit) involves evaluating packaging systems to determine their ability to protect the stability and sterility of pharmaceutical products. The container closure integrity testing method simulates the natural stress conditions that the containers are likely to be exposed to during storage, handling or transportation. Compromised container closure integrity can result in contamination, moisture ingress, or loss of. You should also read this: Does Whippets Show Up On Drug Test

Container Closure Integrity Testing CCIT Parenterals Vacuum - Compromised container closure integrity can result in contamination, moisture ingress, or loss of product sterility. Container closure integrity testing (ccit) is an assay that evaluates the adequacy of container closure systems to maintain a sterile barrier against potential contaminants. Container closure integrity testing (ccit) plays a pivotal role in safeguarding drug products from contamination and degradation. A cartridge container closure. You should also read this: Std Testing Albuquerque Nm

Container Closure Integrity Testing of Sterile Injectable Products - Container closure integrity testing (ccit) plays a pivotal role in safeguarding drug products from contamination and degradation. By verifying that packaging systems are sealed correctly,. Container closure integrity testing (ccit) is a crucial process that ensures the packaging of sterile vials maintains a sterile barrier against potential contaminants. Cormica offers a suite of ccit methods tailored to meet industry standards:. You should also read this: Diesel Injector Pop Tester