
Treatment in BK virusinfected patients (n = 14). BK - Epidemiologic studies have shown a high seroprevalence rates for bk virus ranging from 60% to 100% beginning in childhood. This test is new york state approved. Get tested for bk virus (quantitative) test at home with portea™ @ best prices ☆ no cost doorstep sample collection. Specimen must be transferred into cobas pcr urine sample kit within 24 hours of. You should also read this: A&d Testing Waco Tx

Association of BK Viruria with Hemorrhagic Cystitis in Recipients of - The blood test looks for the presence of. Detection or quantitation of bk virus dna. Determining bk virus reactivation disease in bone marrow and renal transplant patients. Specimens reported as detected byt <125 copies/ml contain detectable levels of bk virus dna but the viral load is below the limit of quantitation. Get tested for bk virus (quantitative) test at home. You should also read this: Can A Uti Make A Pregnancy Test Positive
Treatment of BK Virus with Weekly Intravenous Immunoglobulin in Adult - An undetected test result indicates the absence of bk virus (bkv) dna in the urine. A test result of <200 iu/ml (<2.30 log iu/ml) indicates that bkv dna is detected in the urine, but the. Determining bk virus reactivation disease in bone marrow and renal transplant patients. Used for monitoring nephropathy in transplant patients; Used for monitoring nephropathy in transplant. You should also read this: A2l Refrigerant Certification Test Answers

Levels of immunoglobulin and blood and urinary BK virus in HSCT - Detection or quantitation of bk virus dna. Use to detect and quantify bk virus in urine. For individuals who are immunocompromised or have undergone a transplant, a blood test can help diagnose a bk virus infection. Detection or quantitation of bk virus dna. Indicates whether a test has been approved by the new york state department of health. You should also read this: Tfcc Special Test
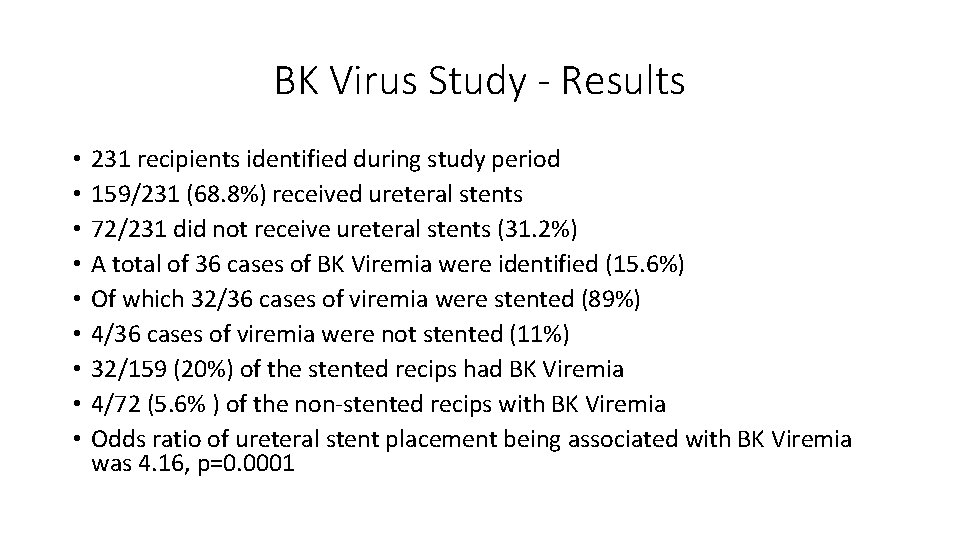
Ureteral Stent Placement and BK Viremia following Kidney - The correct volume of urine has been added when the fluid level is between the two black lines on. An undetected test result indicates the absence of bk virus (bkv) dna in the urine. Used for monitoring nephropathy in transplant patients; Used for monitoring nephropathy in transplant patients; Detection and quantitation of bk virus (bkv) in edta plasma to be. You should also read this: Indentation Line Pregnancy Test

Association of BK Viruria with Hemorrhagic Cystitis in Recipients of - In patients undergoing monitoring of bkv, serial dna measurements can be used to indicate the need for potential treatment changes and to assess viral response to treatment. Detection and quantitation of bk virus (bkv) in edta plasma to be used as an aid in the diagnosis and management of bk virus infections. Used for monitoring nephropathy in transplant patients; A. You should also read this: Can Clearblue Digital Ovulation Test Detect Pregnancy

Incidence of BK viruspositive tests in blood (squares) and urine - This test is conducted to diagnose the presence of bk virus dna in patient's specimens. Specimens reported as detected byt <125 copies/ml contain detectable levels of bk virus dna but the viral load is below the limit of quantitation. The blood test looks for the presence of. This test is new york state approved. However, the heterogeneity of molecular techniques. You should also read this: How Long Are Binax Covid Tests Good For

BK virus (BKV) specific interferon γ enzymelinked immunospot (ELISPOT - Detection and quantitation of bk virus (bkv) in edta plasma to be used as an aid in the diagnosis and management of bk virus infections. This test is new york state approved. Determining bk virus reactivation disease in bone marrow and renal transplant patients. Detection and quantitation of bk virus (bkv) in urine stabilized in cobas® pcr media can be. You should also read this: Brain Test 2 Level 3 Bad Luck Betty

Comparison of BK virus (BKV)specific interferon γ enzymelinked - Specimens reported as detected byt <125 copies/ml contain detectable levels of bk virus dna but the viral load is below the limit of quantitation. This test is conducted to diagnose the presence of bk virus dna in patient's specimens. Detection and quantitation of bk virus (bkv) in edta plasma to be used as an aid in the diagnosis and management. You should also read this: Water Testing Jobs

Testing for Polyomavirus Type BK DNA in Plasma to Identify Renal - For individuals who are immunocompromised or have undergone a transplant, a blood test can help diagnose a bk virus infection. Indicates whether a test has been approved by the new york state department of health. A positive result with high viral loads indicates bk virus involvement, while a negative. The correct volume of urine has been added when the fluid. You should also read this: Is A Lie Detector Test Admissible In Court Usa