
Bioburden Testing ISO 11737 MicroServe Laboratory - Bioburden testing or microbial testing is a quality control process that detects and quantifies microbial contamination of a product at different stages of. Find out the methods, media, and standards used for. Find out the methods, challenges, and services offered by pacific biolabs, a. All batches manufactured are to be sampled for filled container bioburden testing. Microbial contamination poses a. You should also read this: Cremaster Reflex Test

Bioburden Testing - What is the iso 11737 bioburden test? These guidelines aimed to provide a systematic approach to assess the microbial load in pharmaceutical raw materials, finished products, and medical devices by counting the. Find out the methods, challenges, and services offered by pacific biolabs, a. Microbial contamination poses a major safety. It is performed on any product that requires control and/or. You should also read this: Azella Practice Test

Bioburden Testing and Sterility Testing of Medical Devices - It helps to figure out the correct way of sterilizing the raw materials and water used. Bioburden testing is a critical step in the manufacturing process of medical devices. Adhering to gmp standards for bioburden testing helps manufacturers maintain product quality and meet regulatory requirements. Find out the methods, challenges, and services offered by pacific biolabs, a. Find out the. You should also read this: Test Alternator Output
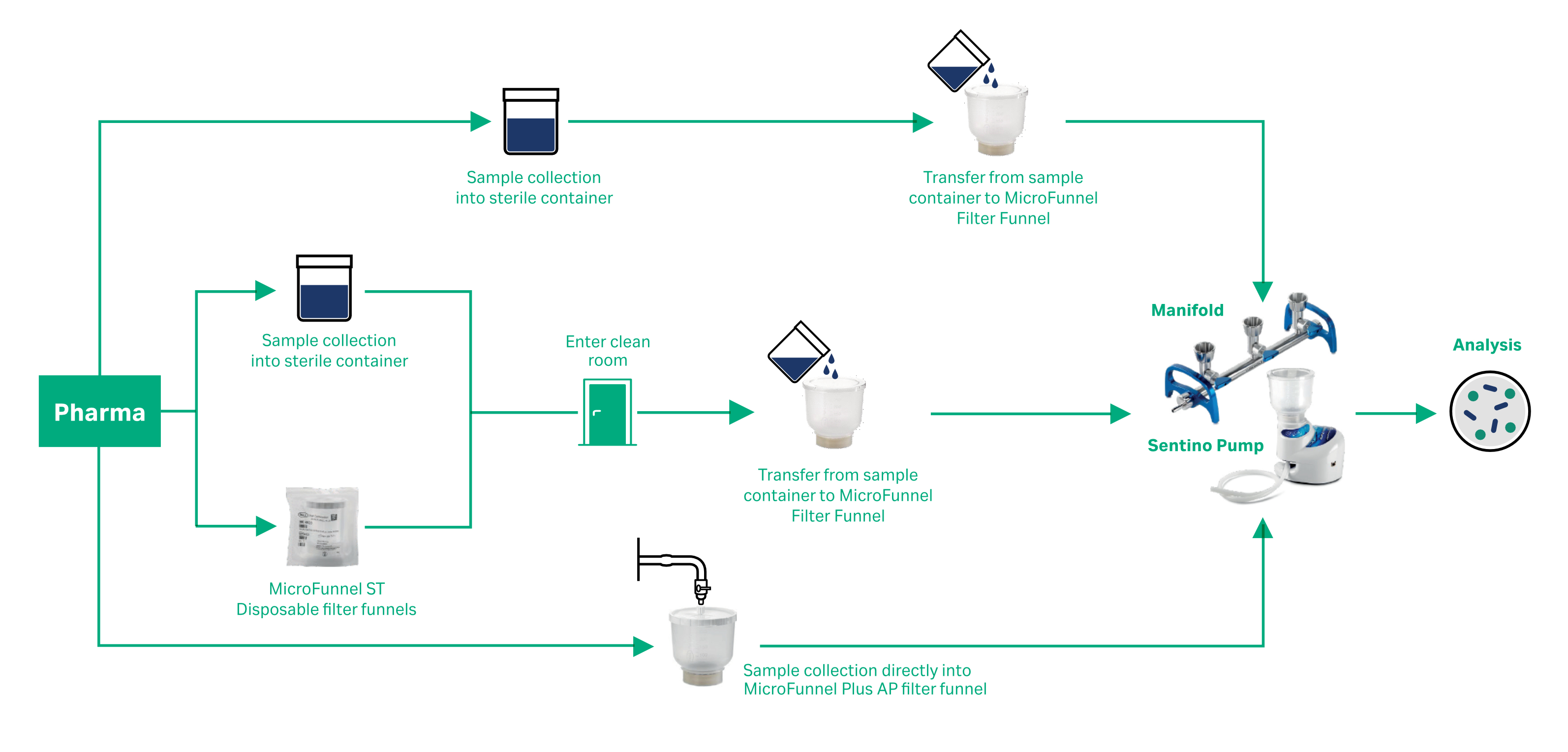
Pharmaceutical bioburden and microbial limits testing Cytiva - The purpose of this standard operating procedure (sop) is to provide guidelines and procedures for determining the microbial bioburden in raw materials or finished products within the. The bioburden testing methods isolate and enumerate viable microorganisms before sterilizing any materials and products. Find out the methods, media, and standards used for. Learn how to perform bioburden and sterility testing of. You should also read this: Beta Test Example

Guide to Bioburden Testing in Medical Device Manufacturing - It helps to figure out the correct way of sterilizing the raw materials and water used. Find out the methods, media, and standards used for. Bioburden testing is the quality control process used to detect and quantify microorganisms like bacteria, fungi, and mold. Find out the methods, challenges, and services offered by pacific biolabs, a. What is the iso 11737. You should also read this: Enneagram Test With Instinctual Variant

PPT Microbiological Testing PowerPoint Presentation, free download - The bioburden test determines the total number of viable microorganisms in or on a medical device, container, or component. These testing methods have many different purposes in different laboratories, which are as follows: It is performed on any product that requires control and/or. It helps to figure out the correct way of sterilizing the raw materials and water used. Find. You should also read this: One Hit Of Delta-8 Drug Test

Bioburden Test for Medical Device - All batches manufactured are to be sampled for filled container bioburden testing. Adhering to gmp standards for bioburden testing helps manufacturers maintain product quality and meet regulatory requirements. 4.1.1 predetermined units are to be randomly selected immediately prior to the autoclaving of the. The bioburden testing methods isolate and enumerate viable microorganisms before sterilizing any materials and products. Bioburden testing. You should also read this: Mandrel Test Equipment

Difference Between BIOBURDEN TEST AND MICROBIAL LIMIT TEST YouTube - 4.1.1 predetermined units are to be randomly selected immediately prior to the autoclaving of the. Adhering to gmp standards for bioburden testing helps manufacturers maintain product quality and meet regulatory requirements. Microbial contamination poses a major safety. Bioburden testing is a critical step in the manufacturing process of medical devices. Learn what bioburden testing is, why it is important, and. You should also read this: Equate Faint Line Pregnancy Test
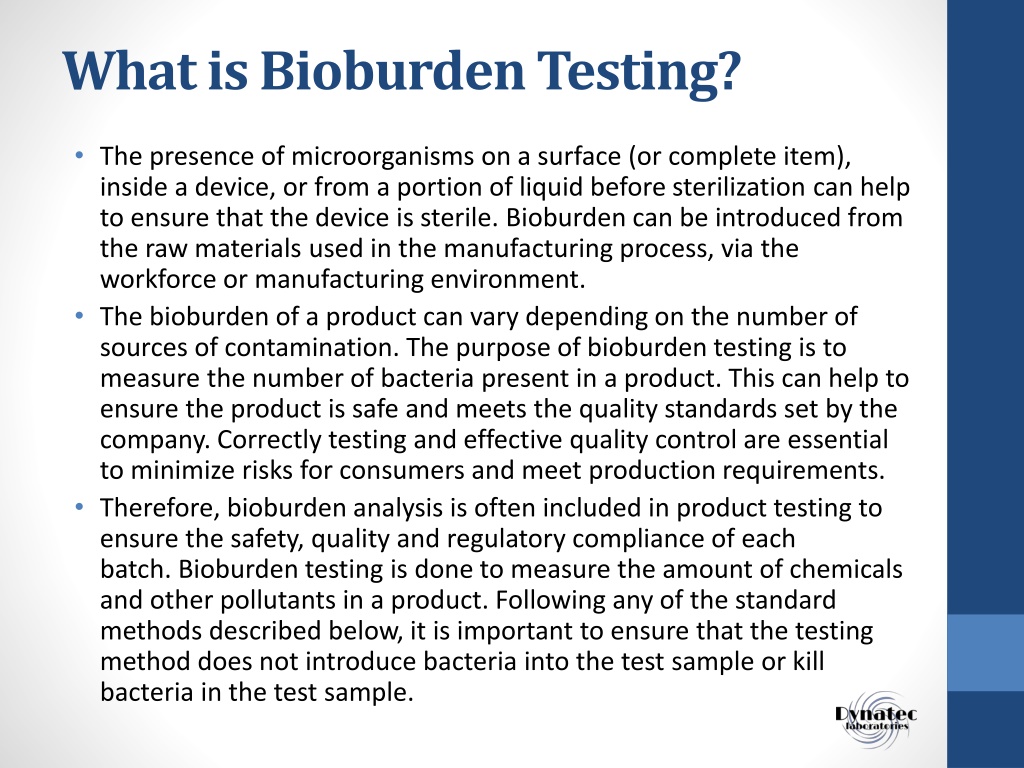
PPT Importance of Bioburden and Testing PowerPoint - Learn what bioburden testing is, why it is important, and how it is conducted for pharmaceutical and medical products. The bioburden testing methods isolate and enumerate viable microorganisms before sterilizing any materials and products. Bioburden testing is the quality control process used to detect and quantify microorganisms like bacteria, fungi, and mold. All batches manufactured are to be sampled for. You should also read this: Cpt Code For Rapid Flu Test

Bioburden Test for Medical Device - 4.1.1 predetermined units are to be randomly selected immediately prior to the autoclaving of the. What is the iso 11737 bioburden test? These testing methods have many different purposes in different laboratories, which are as follows: The bioburden testing methods isolate and enumerate viable microorganisms before sterilizing any materials and products. Find out the methods, challenges, and services offered by. You should also read this: Framingham Cmsc Road Test Site