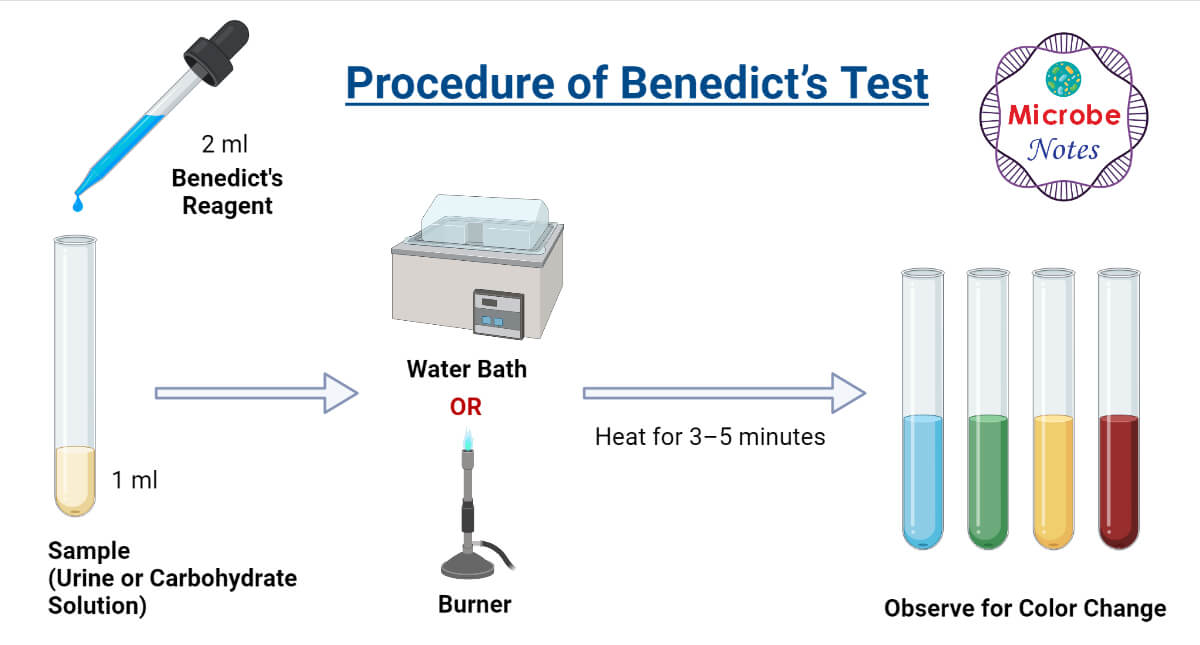
Benedict’s Test Principle, Procedure & Practical Uses - They can reduce cupric ions (cu 2+) to cuprous form (cu +), which is responsible for the change in color of the reaction mixture. It involves the addition of benedict’s reagent, which is a mixture of sodium citrate, sodium carbonate, and copper (ii) sulfate, to a solution containing reducing sugars. This test plays a crucial role in biological, medical, and. You should also read this: Tire Penny Test

Benedict’s Test - Discover its composition, how it works, and why it's preferred over fehling's solution for glucose detection. This test plays a crucial role in biological, medical, and food industries for identifying carbohydrates that contain free ketone or. Benedict’s test detects reducing sugars (sugars having a free reactive carbonyl group). 5h 2 o), sodium citrate (na 3 c 6 h 5 o. You should also read this: Bfm Blood Test

Benedict's test Principle, Procedure, and Uses DewWool - Therefore, simple carbohydrates containing a free ketone or aldehyde functional group can be identified with this test. The presence of the alkaline sodium carbonate converts the sugar into a strong reducing agent called enediols. In this article, we will learn about benedict's test, procedure of benedict’s test, preparation of benedict’s reagent, and others in detail. During the reaction, enediols reduce. You should also read this: Test Tube Mewtwo Psa 10
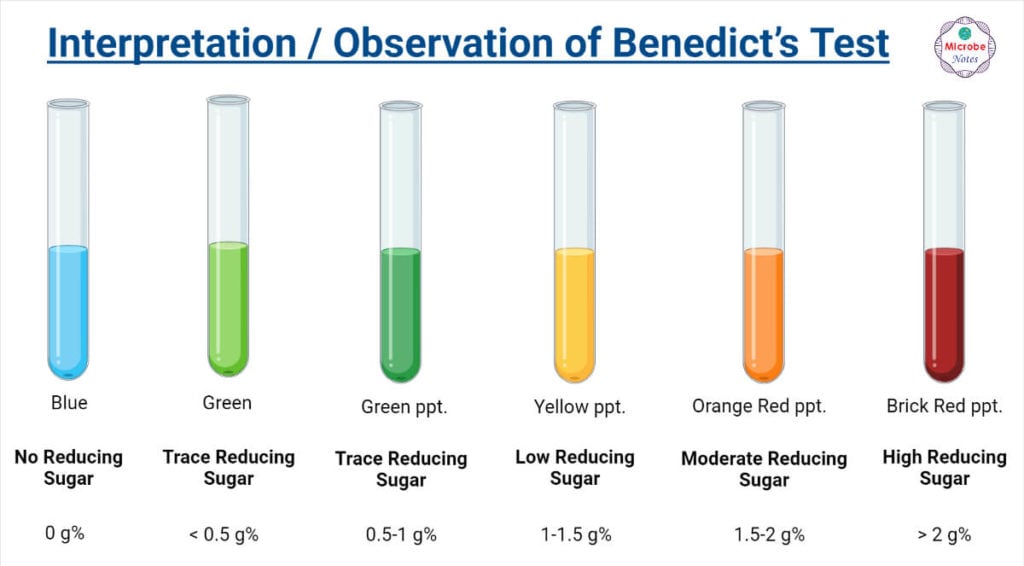
Benedict S Test Principle Preparation Procedure And R vrogue.co - The reducing sugars are capable of reducing copper (ii) ions to copper (i) ions, resulting in a color change that indicates the concentration of reducing sugars. Benedict’s test is used to test for simple carbohydrates. They can reduce cupric ions (cu 2+) to cuprous form (cu +), which is responsible for the change in color of the reaction mixture. Benedict’s. You should also read this: Mpre Test Prep
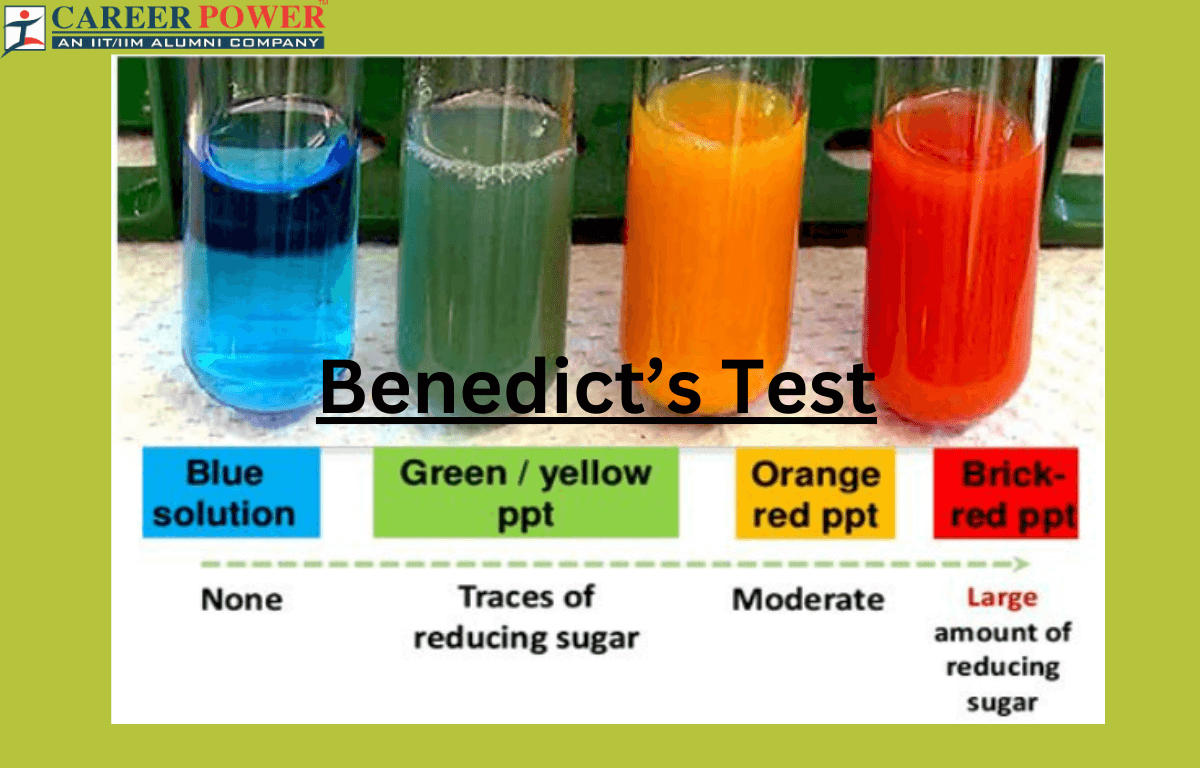
Benedict's Test Reagent, Composition, Principal and Uses - The benedict test is used to detect simple carbohydrates. Therefore, this test can be used to identify simple carbohydrates. The reducing sugars are capable of reducing copper (ii) ions to copper (i) ions, resulting in a color change that indicates the concentration of reducing sugars. During the reaction, enediols reduce the cupric ions (cu 2+) of cuso 4 from benedict’s. You should also read this: Practice Parapro Test

Benedict's test Qualitative test in carbohydrates Urine sugar test - During the test, the sugars give electrons to the cu2+ ions in benedict’s reagent, reducing them to cu+ ions. Look for the development of brick red precipitate. During the reaction, enediols reduce the cupric ions (cu 2+) of cuso 4 from benedict’s reagent to cuprous ions (cu +) which form a yellow precipitate of cuprous hydroxide or a red precipitate. You should also read this: Cannabis Testing Solutions Maldi-based Instruments And Solutions

Benedict’s Test Principle, Procedure, Uses, and Limitation Microbe - The reducing sugars are capable of reducing copper (ii) ions to copper (i) ions, resulting in a color change that indicates the concentration of reducing sugars. Reducing sugars possess a free aldehyde or ketone functional group and can reduce other substances. Benedict’s test is a chemical test that can be used to check for the presence of reducing sugars in. You should also read this: Iodine Level Test

Benedict's Test Principle, Procedure, Preparation of Benedict's - It involves the addition of benedict’s reagent, which is a mixture of sodium citrate, sodium carbonate, and copper (ii) sulfate, to a solution containing reducing sugars. Take 1ml of distilled water in another tube as control. The test is based on benedict’s reagent (also known as benedict’s solution. 5h 2 o), sodium citrate (na 3 c 6 h 5 o. You should also read this: Test Scorer Remote
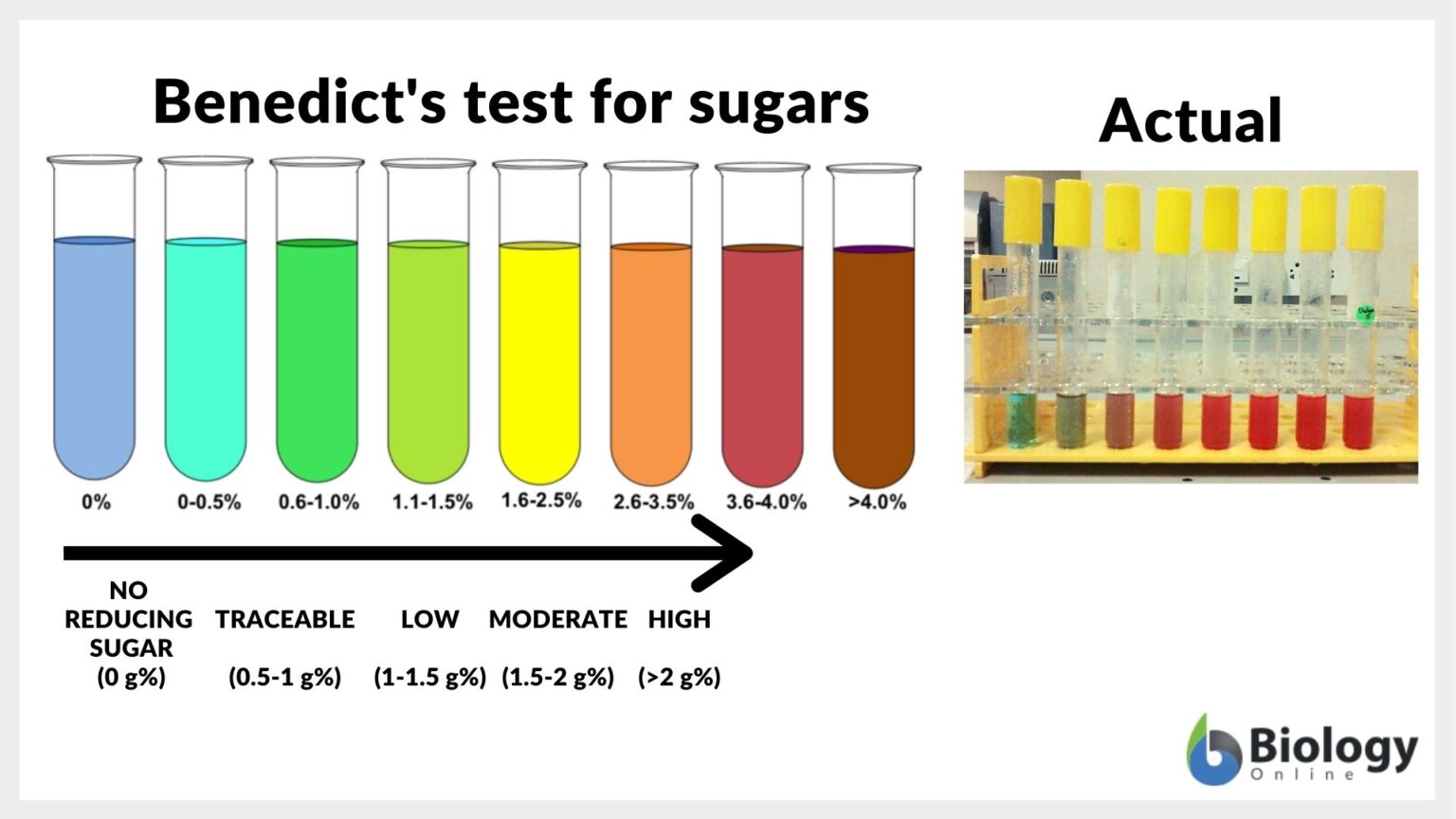
Reducing sugar Definition and Examples Biology Online Dictionary - Benedict's test is a simple yet powerful chemical assay used to detect the presence of reducing sugars in a sample. It is a qualitative chemical test that detects reducing sugars in a given sample. This test plays a crucial role in biological, medical, and food industries for identifying carbohydrates that contain free ketone or. Discover its composition, how it works,. You should also read this: Fac 093 Test Answers

Benedict’s test Definition, Principle, Uses, and Reagent - This is the basis of benedict’s test. Look for the development of brick red precipitate. Tests that use this reagent are called benedict's tests. Therefore, simple carbohydrates containing a free ketone or aldehyde functional group can be identified with this test. Benedict’s test is used to test for simple carbohydrates. You should also read this: Polygraph Test For Firefighters