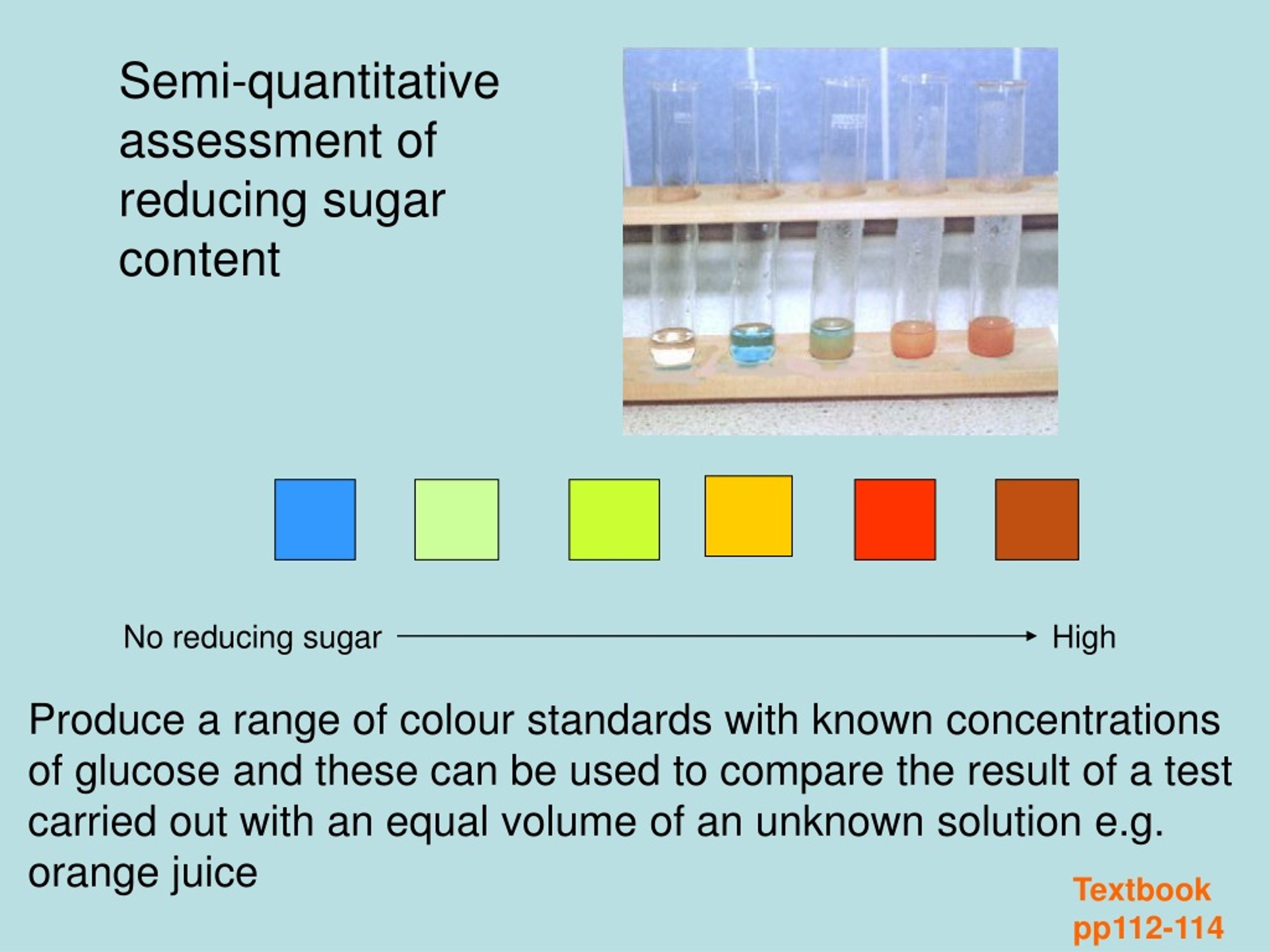
PPT Testing for Reducing sugars Benedict’s Test PowerPoint - Reducing sugars under alkaline condition tautomerise and form enediols. Benedict’s test is performed by heating the reducing sugar solution with benedict‘s reagent. This is because benedict’s test produces a insoluble red precipitate of copper (i) oxide. Benedict’s test identifies reducing sugars, which have a free aldehyde or ketone group. Benedict’s solution can be used to test for the presence of. You should also read this: Dilated Pupils Eye Test
Benedict’s Test Principle, Procedure & Practical Uses - Enediols are powerful reducing agents. The benedict’s test is grounded in the principle of detecting reducing sugars through their ability to reduce copper (ii) ions to copper (i) ions under alkaline conditions. The process of shifting of a hydrogen atom from one carbon atom to another in alkaline condition to produce enediols is known as tautomerization or enolization. These sugars. You should also read this: Tamu Soil Testing
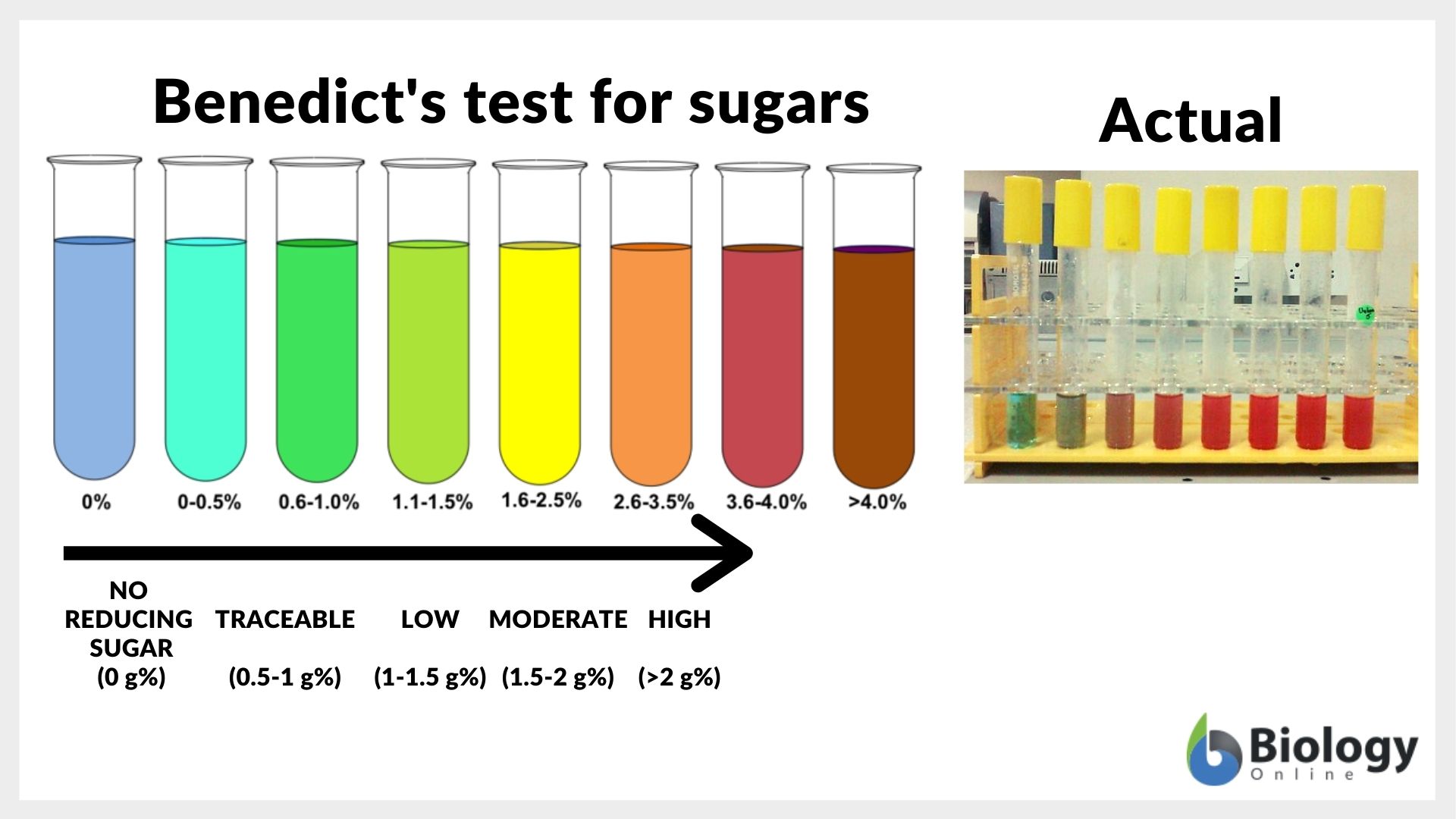
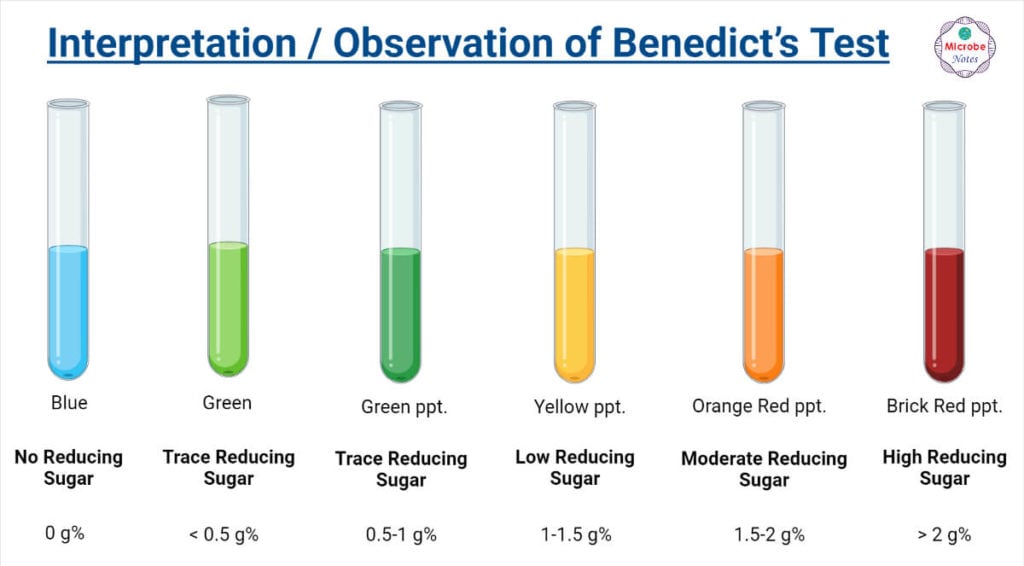
Reducing sugar Definition and Examples Biology Online Dictionary - When you remove the water bath you can tell if it is a reducing sugar dependent on the colour change: During the test, the sugars give electrons to the cu2+ ions in benedict’s reagent, reducing them to cu+ ions. The benedict’s test identifies reducing sugars (monosaccharide’s and some disaccharides), which have free ketone or aldehyde functional groups. These include monosaccharides. You should also read this: Free Radon Test Kit Missouri

Benedict's Test for Reducing Sugars Amanda Metcalfe - During the test, the sugars give electrons to the cu2+ ions in benedict’s reagent, reducing them to cu+ ions. The process of shifting of a hydrogen atom from one carbon atom to another in alkaline condition to produce enediols is known as tautomerization or enolization. When benedict’s reagent is added to a sample containing reducing sugars, such as glucose or. You should also read this: Question Mark First Response Pregnancy Test

Premium Vector Benedict's Test for Reducing Sugars - These include monosaccharides like glucose and fructose and. Reducing sugars under alkaline condition tautomerise and form enediols. A useful thing about the benedict’s test is that it is quantitative. The benedict test identifies reducing sugars (monosaccharides and some disaccharides) that have free ketone or aldehyde functional groups. They can reduce cupric ions (cu 2+) to cuprous form (cu +), which. You should also read this: Annual Osd Records And Information Management Training Post Test
Benedict’s Test Principle, Procedure & Practical Uses - The benedict’s test is grounded in the principle of detecting reducing sugars through their ability to reduce copper (ii) ions to copper (i) ions under alkaline conditions. Benedict’s test is based on the principle that under alkaline conditions reducing sugar forms enediols which are powerful reducing agents. Reducing sugars are carbohydrates having free aldehyde or ketone functional groups in their. You should also read this: Iq Test 120 Score

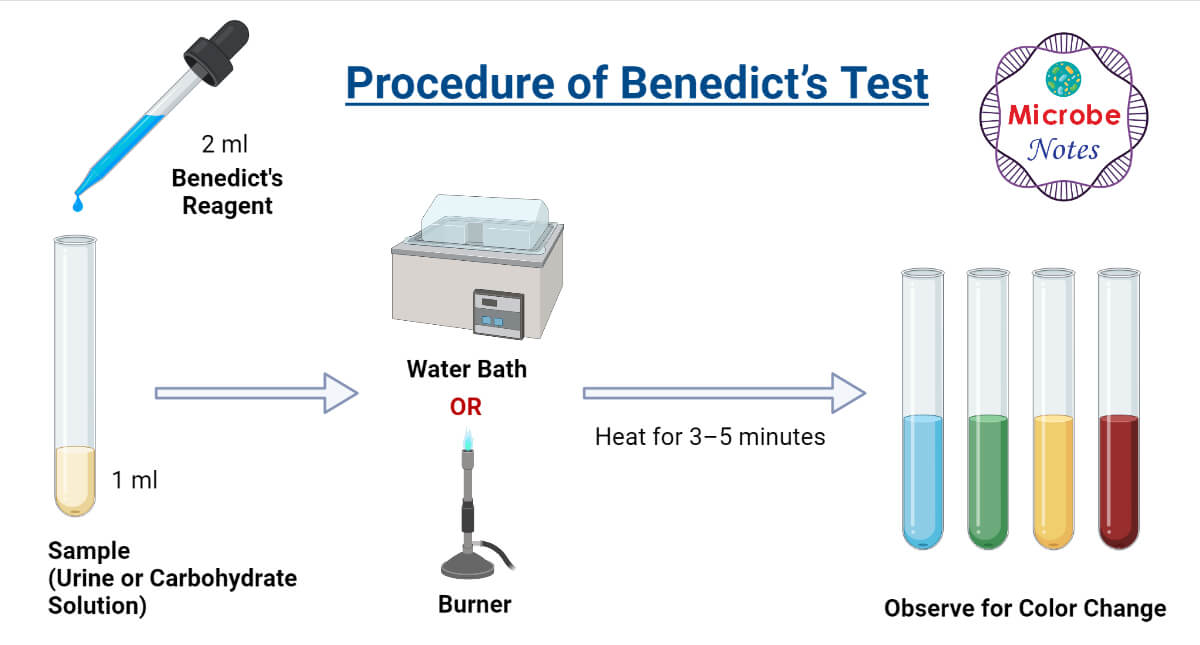
Biology Lab Report Food Tests Brilliant Biology Student - This is the basis of benedict’s test. The primary application of benedict’s test is to detect the presence of simple carbohydrates in an unidentified analyte. This results in a color change, which is indicative of the concentration of reducing sugars in the sample. When benedict's reagent is heated with the reducing sugar, benedict's test is carried out. The sugar is. You should also read this: Does Nitrous Show Up In Urine Test

Benedict’s Test - Enediols are powerful reducing agents. Examples include glucose, fructose, lactose, and maltose. Benedict's test and fehling's test are two common tests for reducing sugars. Reducing sugars possess a free aldehyde or ketone functional group and can reduce other substances. This means that the test can be measured for how much reducing sugar is present. You should also read this: Biology Unit 1 Practice Test

Benedict’s test Definition, Principle, Uses, and Reagent - The process of shifting of a hydrogen atom from one carbon atom to another in alkaline condition to produce enediols is known as tautomerization or enolization. Benedict’s test is a simple chemistry test used to detect reducing sugars. Benedict's test and fehling's test are two common tests for reducing sugars. This test can be used to check for reducing sugars. You should also read this: Permit Test Online Oregon
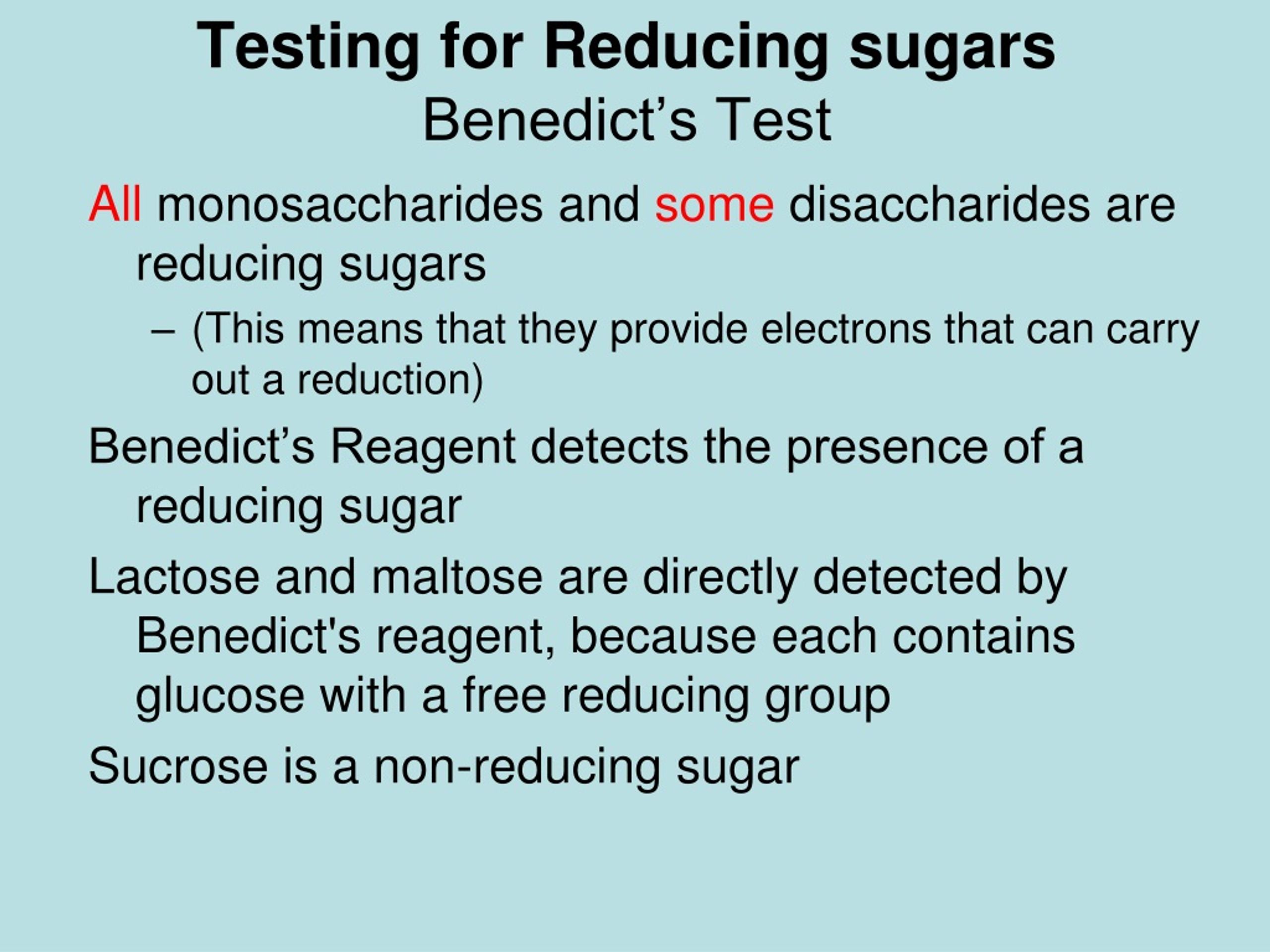
PPT Testing for Reducing sugars Benedict’s Test PowerPoint - The benedict solution contains milder alkali, na 2 co 3 to maintain alkaline conditions. A useful thing about the benedict’s test is that it is quantitative. They can reduce cupric ions (cu 2+) to cuprous form (cu +), which is responsible for the change in color of the reaction mixture. Reducing sugars possess a free aldehyde or ketone functional group. You should also read this: Minuteman Iii Test Launch