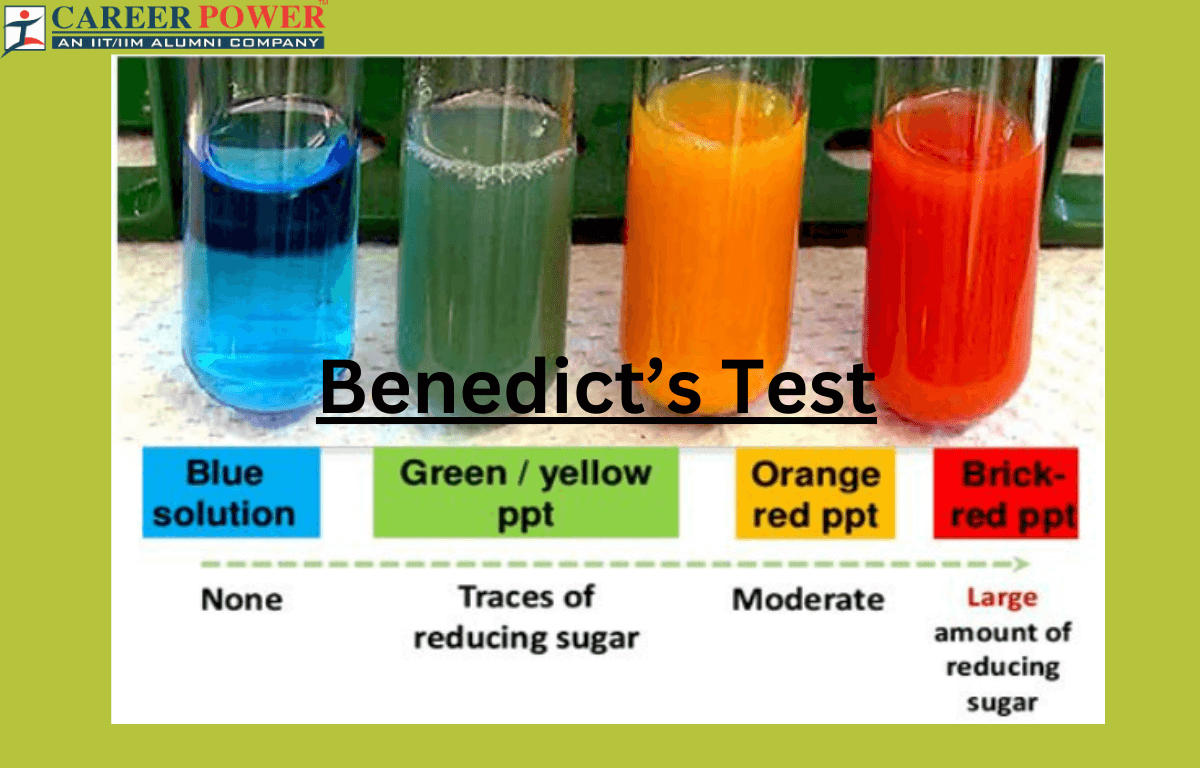
Benedict's Test Reagent, Composition, Principal and Uses - The benedict solution contains milder alkali, na 2 co 3 to maintain alkaline conditions. Benedict’s reagent, also known as benedict’s solution is used in benedict’s test for detecting simple sugars such as glucose. Reducing sugars are carbohydrates having free aldehyde or ketone functional groups in their molecular structure. Benedict’s solution contains milder alkali na2co3. The benedict’s test identifies reducing sugars. You should also read this: Tachs Exam Practice Test

Benedict's Test Principle, Procedure, Preparation of Benedict's - It is a type of test done to detect the reducing sugar (they are carbohydrates that have aldehyde or ketone functional group in molecular structure, which include monosaccharides like glucose and fructose and disaccharides like maltose and lactose) Benedict's reagent (often called benedict's qualitative solution or benedict's solution) is a chemical reagent and complex mixture of sodium carbonate, sodium citrate,. You should also read this: Methylation Test Quest Diagnostics

Benedict’s Test - The benedict solution contains milder alkali, na 2 co 3 to maintain alkaline conditions. Benedict's reagent (often called benedict's qualitative solution or benedict's solution) is a chemical reagent and complex mixture of sodium carbonate, sodium citrate, and copper(ii) sulfate pentahydrate. Reducing sugars are carbohydrates that can reduce certain compounds due to their free aldehyde or ketone groups. Benedict’s test is. You should also read this: Wdl Test Menu
Benedict’s Test Principle, Procedure & Practical Uses - Benedict’s test is a simple chemistry test used to detect reducing sugars. It is a bright blue solution that is prepared by mixing copper sulfate pentahydrate, sodium carbonate, and sodium citrate in distilled water. Benedict’s reagent, also known as benedict’s solution is used in benedict’s test for detecting simple sugars such as glucose. Benedict’s test is a qualitative laboratory test. You should also read this: Does Propofol Show Up On A Drug Test

Benedict's test Principle, Procedure, and Uses DewWool - The benedict's test is used to detect simple carbohydrates. Benedict’s test is a qualitative laboratory test used to determine the presence of reducing sugars in each solution. Benedict’s reagent, also known as benedict’s solution is used in benedict’s test for detecting simple sugars such as glucose. The benedict solution contains milder alkali, na 2 co 3 to maintain alkaline conditions.. You should also read this: Wais 4 Test Online

Benedicts Test for Reducing Sugars TrevonhasMorse - Benedict’s test is a simple chemistry test used to detect reducing sugars. Enediols are powerful reducing agents. Benedict’s test is a chemical test that can be used to check for the presence of reducing sugars in a given analyte. It is a bright blue solution that is prepared by mixing copper sulfate pentahydrate, sodium carbonate, and sodium citrate in distilled. You should also read this: How Many Dpo To Test For Pregnancy

Benedict’s test Definition, Principle, Uses, and Reagent - They can reduce cupric ions to cuprous ions which is the basis for benedict’s reaction. Benedict’s test is most commonly used to test for the presence of glucose in urine. It is a type of test done to detect the reducing sugar (they are carbohydrates that have aldehyde or ketone functional group in molecular structure, which include monosaccharides like glucose. You should also read this: Emissions Test San Mateo Albuquerque
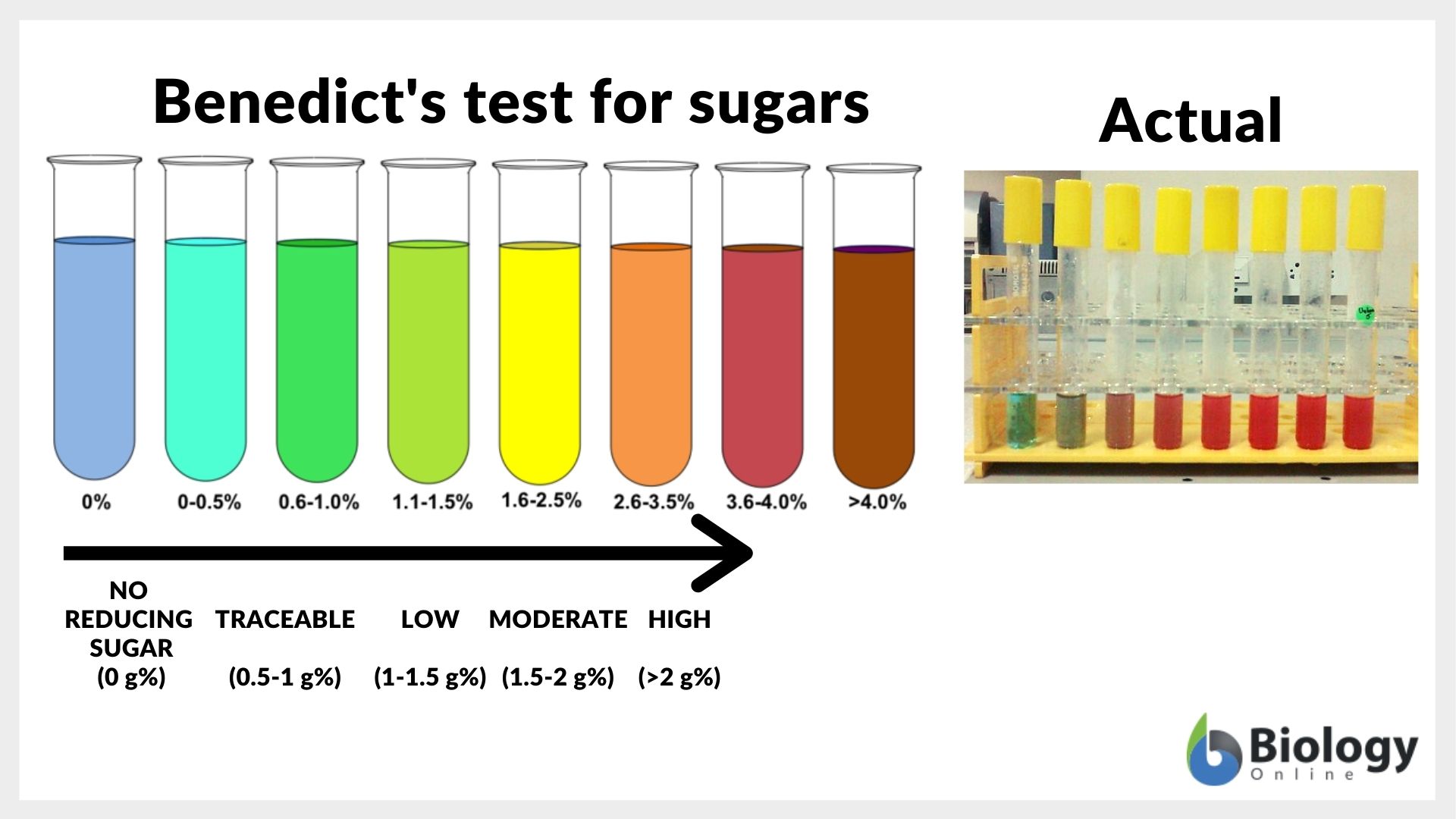
Reducing sugar Definition and Examples Biology Online Dictionary - The cuprous hydroxide during the process of heating is converted to red cuprous oxide. Benedict’s test is based on the principle that under alkaline conditions reducing sugar forms enediols which are powerful reducing agents. Benedict’s test is a biochemical assay used to detect reducing sugars, which are important in biological processes and medical diagnostics. Benedict’s reagent, also known as benedict’s. You should also read this: Can You Take An Edible Before A Blood Test
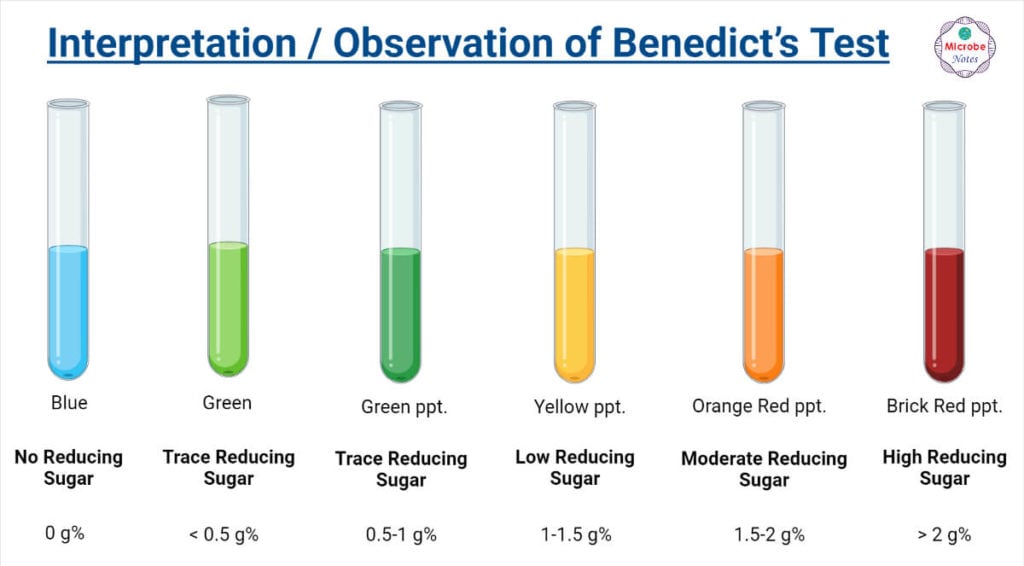
Benedict’s Test Principle, Procedure & Practical Uses - Benedict’s test is a simple chemistry test used to detect reducing sugars. Reducing sugars are carbohydrates that can reduce certain compounds due to their free aldehyde or ketone groups. Benedict’s test is most commonly used to test for the presence of glucose in urine. Enediols are powerful reducing agents. It is a type of test done to detect the reducing. You should also read this: Kb Test In Pregnancy

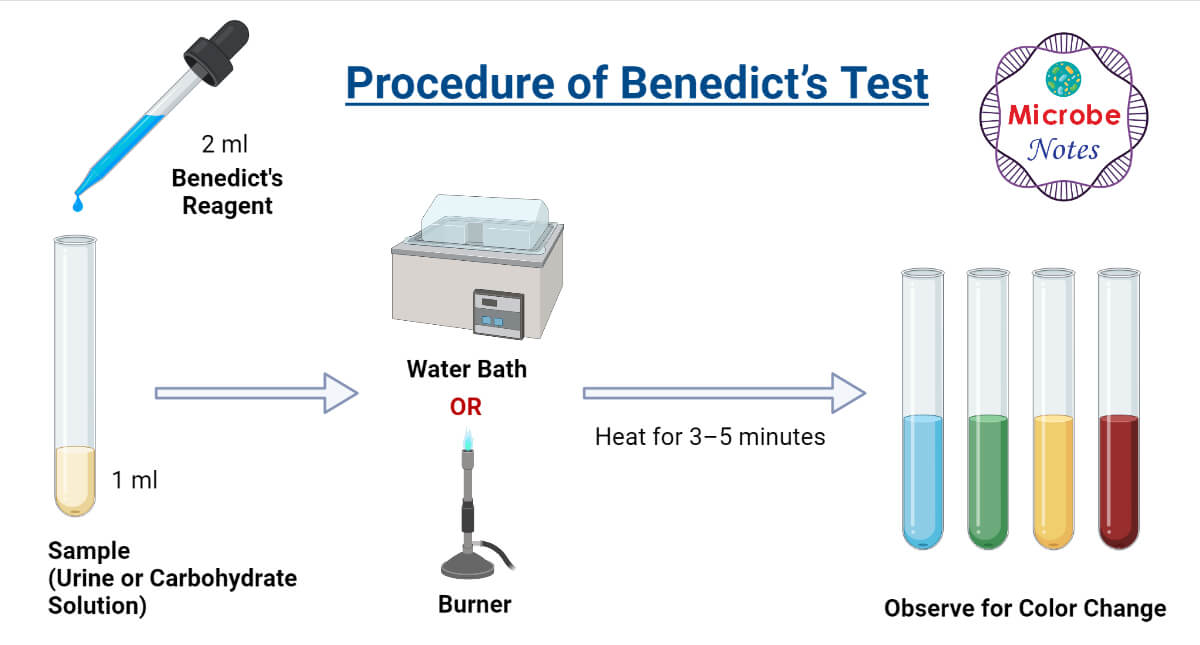
What Is A Positive Test Result For The Benedict's Test at David - Benedict's test is a chemical test used to determine reducing sugar in any solution. The benedict solution contains milder alkali, na 2 co 3 to maintain alkaline conditions. Benedict’s reagent, also known as benedict’s solution is used in benedict’s test for detecting simple sugars such as glucose. Benedict’s test is performed by heating the reducing sugar solution with benedict‘s reagent.. You should also read this: Biohazard Cleaning Services Atp Testing