
Baeyer's test of alkenes unsaturation test Alkene hydrocarbon - The baeyers test is used to test for an unsaturated carbon carbon bond, such as an alkene or alkyne, but not an aromatic carbon carbon bond. Alkenes, for example, are oxidised to glycols, and the permanganate loses its colour: However, if an alkaline reagent is used, compounds having an active hydrogen (carbon acids). Phenols and aryl amines give a positive. You should also read this: Ap Euro Unit 1 Practice Test

Baeyer's test for unsaturation of alkenes and alkynesKMNO4 test for - Carbonyl compounds which decolorize bromine/methylene chloride usually give a. Baeyer’s reagent is an alkaline solution of cold potassium permanganate (kmno4). The baeyer’s test for unsaturation has been used in qualitative organic analysis for a long time. Potassium permanganate (the baeyer test) a second qualitative test for unsaturation, the baeyer test, depends on the ability of potassium permanganate to oxidize the. You should also read this: Does Rare Beauty Test On Animals

A Level Chemistry Revision Organic Chemistry Organic Analysis - The baeyer test for unsaturation uses a potassium permanganate (kmno 4) aqueous solution that, in the presence of alkenes and alkynes, changes from purple to dark. Potassium permanganate (the baeyer test) a second qualitative test for unsaturation, the baeyer test, depends on the ability of potassium permanganate to oxidize the carbon‑carbon. Alcohols with trace impurities give a positive test. The. You should also read this: Fnf Wednesday Infidelity Test

Baeyer's Test for Alkanes - The baeyers test is used to test for an unsaturated carbon carbon bond, such as an alkene or alkyne, but not an aromatic carbon carbon bond. Alkenes, for example, are oxidised to glycols, and the permanganate loses its colour: The reaction involves initial addition of a peroxide to the carbonyl carbon. He proposed the correct formula for indole and achieved. You should also read this: Can I Get My Hormones Tested
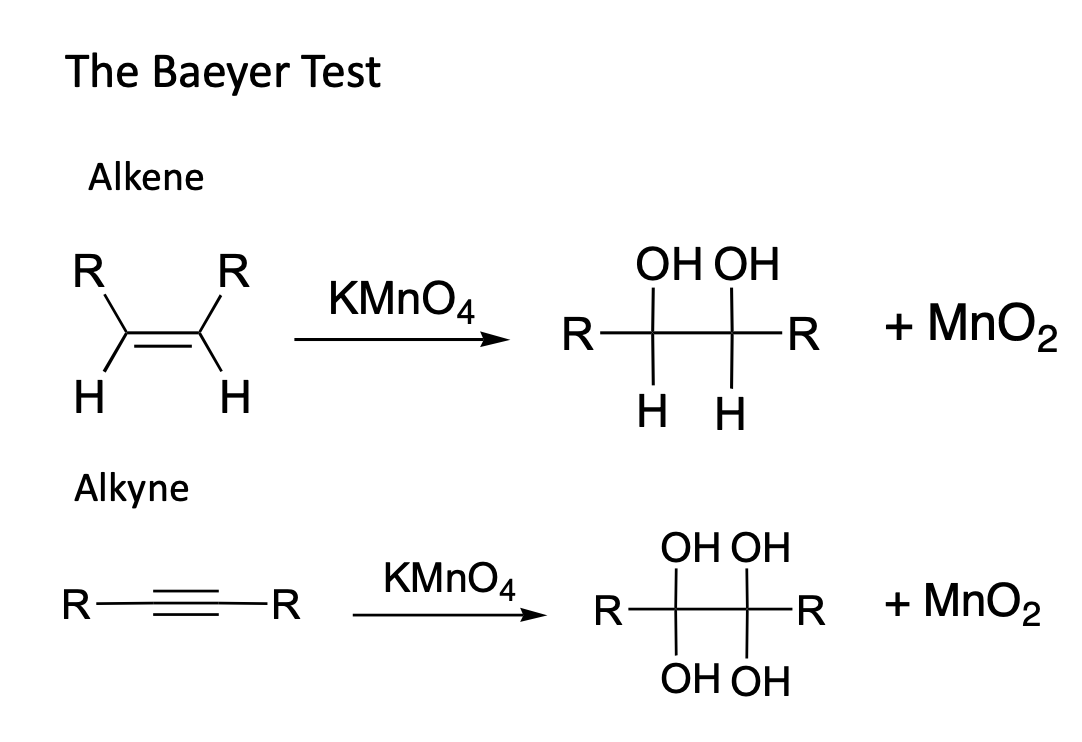
Solved The Baeyer Test Alkene R. R KMnO4 ОН ОН > RTER + MnO, - He proposed the correct formula for indole and achieved the synthesis of indigo. The baeyers test is used to test for an unsaturated carbon carbon bond, such as an alkene or alkyne, but not an aromatic carbon carbon bond. Baeyer’s reagent is an alkaline solution of cold potassium permanganate (kmno4). Baeyer's test is used to detect the presence of unsaturation. You should also read this: California Means Test

Hydroxylation (Baeyer's Test).Professor Aziz Atif YouTube - The resulting adduct undergoes rearrangement to form the ester. It is prepared by dissolving anhydrous sodium carbonate (na 2 co 3) in a kmno 4 solution. Used in qualitative organic analysis, it tests. Baeyer’s reagent is an alkaline solution of cold potassium permanganate (kmno4). Alkenes, for example, are oxidised to glycols, and the permanganate loses its colour: You should also read this: Allen Test Thoracic Outlet Syndrome

Question Video Identifying the Product of the Baeyer Test with Ethene - The baeyers test is used to test for an unsaturated carbon carbon bond, such as an alkene or alkyne, but not an aromatic carbon carbon bond. The reaction involves initial addition of a peroxide to the carbonyl carbon. Baeyer’s reagent is an alkaline solution of cold potassium permanganate (kmno4). Baeyer’s reagent is an alkaline solution of cold potassium permanganate (kmno4),. You should also read this: Nipp Testing Near Me

Baeyer's Test for Alkanes OlivertaroRivera - The baeyer’s test for unsaturation has been used in qualitative organic analysis for a long time. Baeyer's test is used to detect the presence of unsaturation in organic compounds, such as alkenes or alkynes. Baeyer test a test for unsaturated compounds in which potassium permanganate is used. Baeyer’s reagent is an alkaline solution of cold potassium permanganate (kmno4). Used in. You should also read this: 30 Nursing Ceus No Test
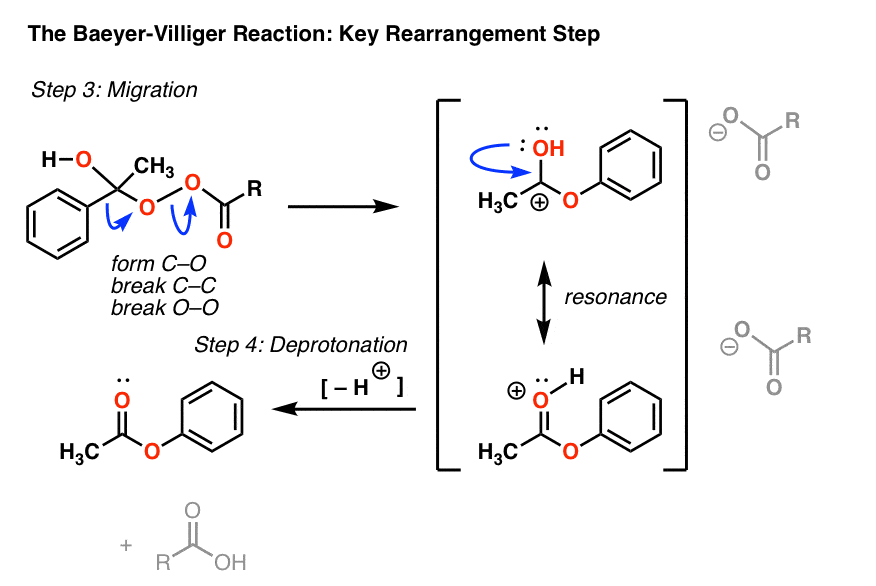
Reduction of Nitro Groups, The BaeyerVilliger, and Protection of Amines - Carbonyl compounds which decolorize bromine/methylene chloride usually give a. He proposed the correct formula for indole and achieved the synthesis of indigo. Baeyer’s reagent is an alkaline solution of cold potassium permanganate (kmno4), known for its strong oxidizing properties. The resulting adduct undergoes rearrangement to form the ester. Alcohols with trace impurities give a positive test. You should also read this: Concentra Drug Test Price

Baeyer’s Reagent Definition, Preparation, and Reaction - Alkaline potassium permanganate (kmno 4) solution is called as bayer's reagent. It is a useful oxidizing agent and is used in qualitative organic analysis to test for the presence. The reaction involves initial addition of a peroxide to the carbonyl carbon. Used in qualitative organic analysis, it tests. The baeyer test for unsaturation uses a potassium permanganate (kmno 4) aqueous. You should also read this: Asbestos Testing Pasadena