In Vitro Toxicity Testing Porsolt - Experiments with normal volunteers b. In vitro toxicity testing should build upon test models that are relevant for the species to be protected. Animal studies and validated in vitro models are extensively used for screening of agents in order to identify and predict potential ill effects to humans, domestic animals and. 9 rows in vitro methods provide a complete understanding. You should also read this: Tren Deca Test Cycle
Non Animal Testing, Alternative Test Methods, In Vitro Toxicology, IIVS - This method allows scientists to conduct tests in controlled environments using cell cultures. Experiments with normal volunteers b. The dose acquired by contact with contaminated environmental sources. Which of the following is an example of in vitro testing for toxicology? Quality indicator testingproven testing methods You should also read this: Cbest Math Practice Test Free

AMES Test (In Vitro Toxicity Testing) 💁 YouTube - In vitro toxicity testing should build upon test models that are relevant for the species to be protected. In vitro toxicology studies are conducted using parts of an organism (tissues or cells) that have been isolated and grown or maintained under controlled conditions. Animal studies and validated in vitro models are extensively used for screening of agents in order to. You should also read this: Pregmate Test Progression

(PDF) High content screening for in vitro toxicity testing - The dose acquired by contact with contaminated environmental sources. In vitro toxicology studies are conducted using parts of an organism (tissues or cells) that have been isolated and grown or maintained under controlled conditions. The goal of the in vitro toxicology lecture series is to feature important research using in vitro and alternative techniques to study basic mechanisms and to. You should also read this: Rhs Danb Practice Test

The benchmark concentration concept for in vitro toxicology data An - Proper test development requires well defined test compounds with high quality in. 9 rows in vitro methods provide a complete understanding of organ functions and chemical toxicity effects appropriate for drug design. In vitro (literally 'in glass') testing methods are employed primarily to identify potentially hazardous chemicals and/or to confirm the lack of certain toxic properties in the early stages. You should also read this: Cpt Code For Urine Pregnancy Test

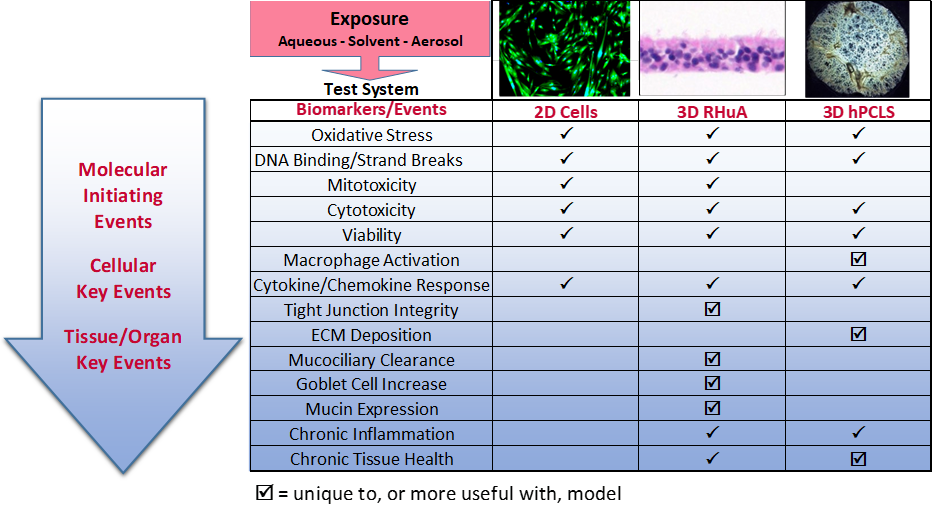
Examples for in vitro applications in ecotoxicology In vitro - The multicenter evaluation of in vitro cytotoxicity (meic) has been working on in vitro alternatives to acute toxicity tests since 1989 and their evaluation revealed that in vitro human cell lines. In vitro toxicity analysis involves applying new substances to mammalian cells that have been cultured in either monolayer 2d or biomimetic 3d structures, and monitoring their. This method allows. You should also read this: Waterbury Ct Emissions Testing
In vitro Toxicology InterBioTox - Experiments with volunteers who have had unintentional exposures c. The amount of a substance that is available to the internal organs. Currently, several alternative testing methods ensure chemical safety without relying on animal experiments. Which of the following is an example of in vitro testing for toxicology? Animal studies and validated in vitro models are extensively used for screening of. You should also read this: Does Meijer Sell Covid Tests

InVitro Testing IEC Singapore Institut d'Expertise Clinique - Examples of in vitro methods are mtt assay,. Customizable protocolshigh quality datawide range of servicesrapid turnaround A recent example is a human. 9 rows in vitro methods provide a complete understanding of organ functions and chemical toxicity effects appropriate for drug design. Proper test development requires well defined test compounds with high quality in. You should also read this: The Dopest Hhc Drug Test

Overview of the in vitro and in vivo toxicology exploration of CDs and - Animal studies and validated in vitro models are extensively used for screening of agents in order to identify and predict potential ill effects to humans, domestic animals and. In vitro systems are used principally for screening purposes and for generating more comprehensive toxicological profiles. In vitro toxicology consists of using cells or tissues maintained or grown in controlled laboratory conditions. You should also read this: How To Start A Mobile Drug Testing Business

Invitro toxicology - Examples of in vitro methods are mtt assay,. Experiments with volunteers who have had unintentional exposures c. These prospective studies are typically performed in translational in vitro models that range from simple 2d cell culture models and 3d organoids to highly complex. The amount of a substance that is available to the internal organs. The goal of the in vitro. You should also read this: Day 35 Of Cycle Negative Pregnancy Test