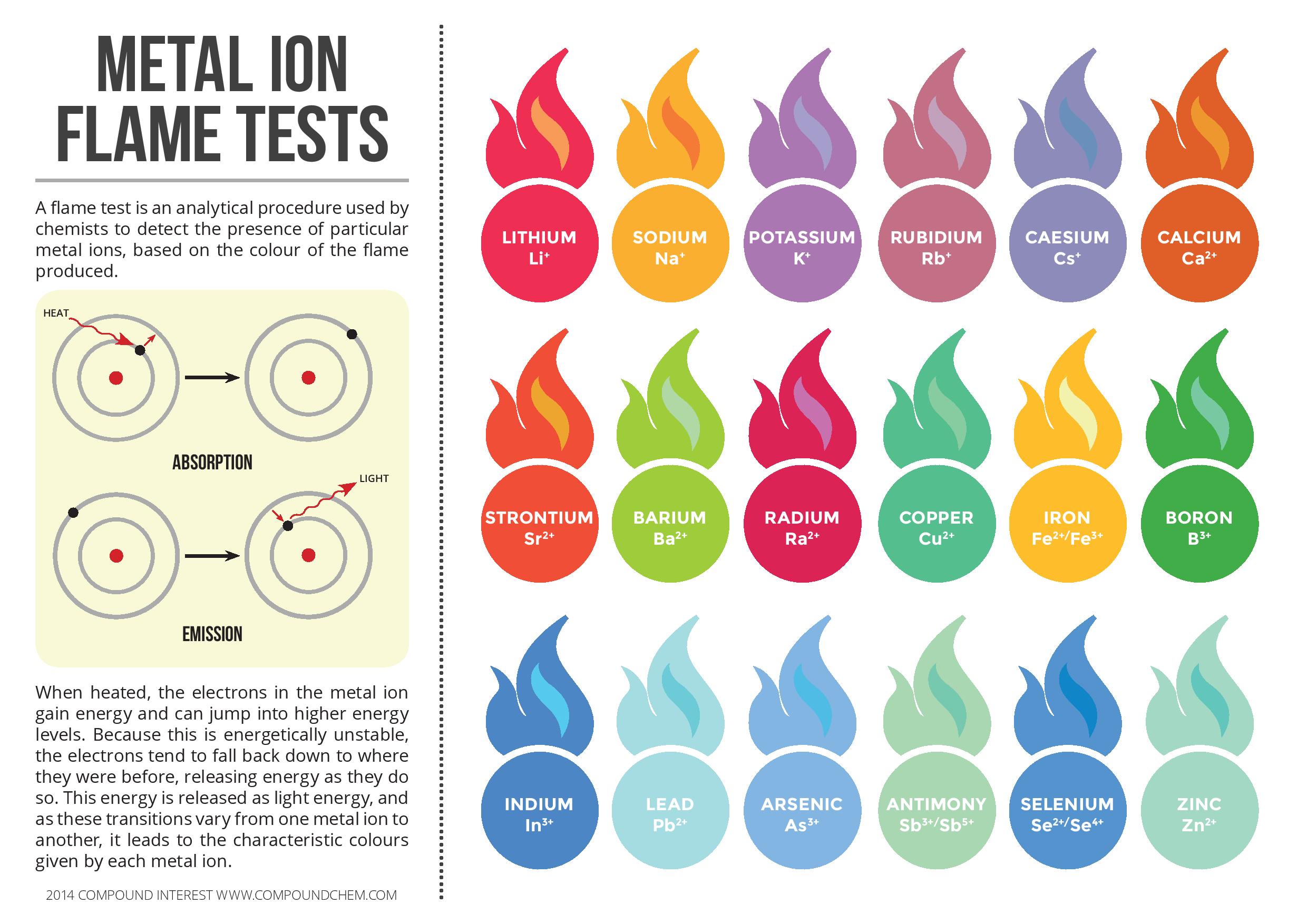
Procedure Of Flame Test - Remove the flame, and then hold a piece of damp red litmus. Transition element ions with partially filled d orbitals tend to release colored light. These photons have a frequency (light color) that is a characteristic of the element. We can identify some common metal cations using a flame test (which is a practical demonstration of atomic emission spectroscopy). Flame. You should also read this: Walgreens Freestyle Lite Test Strips

Flame test of ammonium ion YouTube - Ammonium compounds do not produce a distinct color in a flame test. Practice the flame test under the supervision of a chemistry teacher. Dropping to a more stable energy state involves the release of photons. However, not all elements release. Instrumental methods of analysis are faster, and more accurate and more sensitive than simple chemical. You should also read this: State Police Drivers Test

Solved Identification of an unknown UNKNOWN A Flame test - The basic premise is that heat from a flame gives atoms enough energy that their electrons become excited. Ammonium salts release ammonia gas and water vapor when heated,. Al, k, li, mg, na, ca, ba, sr, zn are colorless aqueous ions and most of their solid salts are white. Add dilute naoh solution and heat gently; Instead, they usually have. You should also read this: Homocysteine Levels Test

Atomic Emission Spectroscopy & Flame Test HSC Chemistry Science Ready - Instrumental methods of analysis are faster, and more accurate and more sensitive than simple chemical. Transition element ions with partially filled d orbitals tend to release colored light. We can identify some common metal cations using a flame test (which is a practical demonstration of atomic emission spectroscopy). However, not all elements release. Dropping to a more stable energy state. You should also read this: What Does Pg/mg Mean On Drug Test

Solved UNKNOWNC Flame test Test for ammonium ion Test for - Here's how you can identify ammonium compounds using a basic chemical test: Study with quizlet and memorize flashcards containing terms like what do flame tests do?, outline briefly how to do a flame test:, what are the two main problems with a flame test? Ammonium ions (nh 4+) can be detected in a. This page describes how to do a. You should also read this: Accuplacer Arithmetic Practice Test

Flame Test To identify the metal in a given unknown substance by - Add dilute naoh solution and heat gently; We can identify some common metal cations using a flame test (which is a practical demonstration of atomic emission spectroscopy). Instead, they usually have no visible flame color or may show a pale blue flame due to the presence of. Instrumental methods of analysis are faster, and more accurate and more sensitive than. You should also read this: Failed 1 Hr Glucose Test

Flame Test Colors YouTube - This page describes how to do a flame test for a range of metal ions, and briefly describes how the flame colour arises. However, not all elements release. Instead, they usually have no visible flame color or may show a pale blue flame due to the presence of. We can identify some common metal cations using a flame test (which. You should also read this: Buzzfeed Autism Test
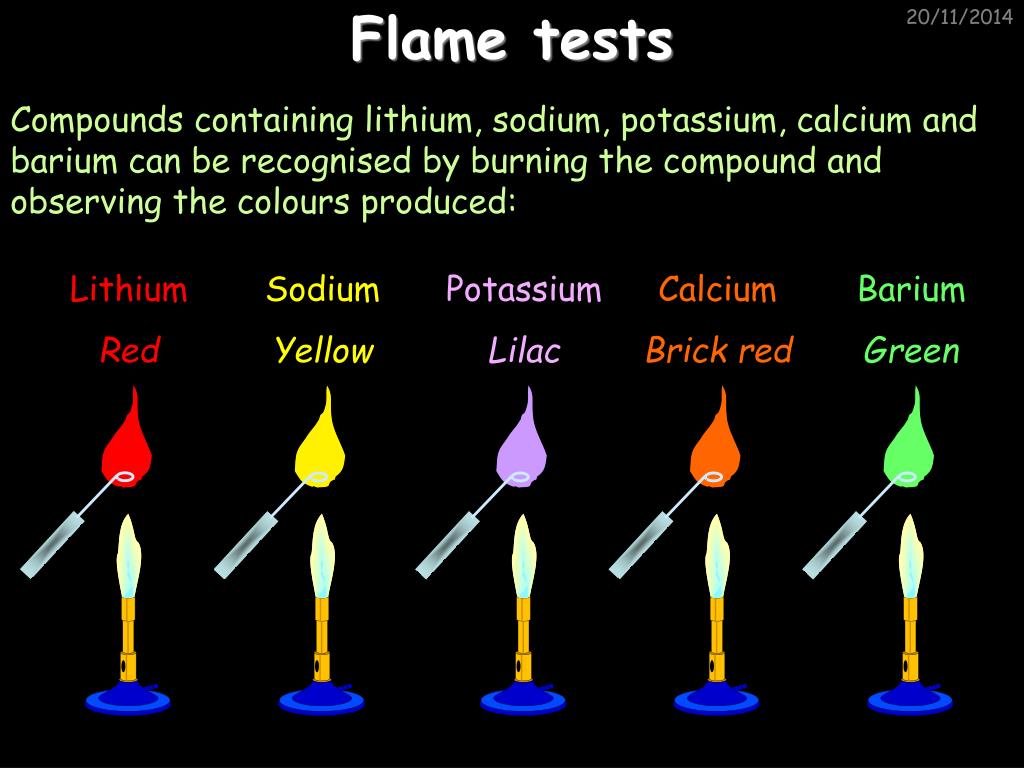
PPT Chemistry in Action PowerPoint Presentation, free download ID - Flame tests are used to identify the presence of a relatively small. Remove the flame, and then hold a piece of damp red litmus. However, not all elements release. We can identify some common metal cations using a flame test (which is a practical demonstration of atomic emission spectroscopy). These photons have a frequency (light color) that is a characteristic. You should also read this: Pbsc Testing Center Lake Worth

Solved UNKNOWN B Flame test Test for ammonium ion Test for - Study with quizlet and memorize flashcards containing terms like what do flame tests do?, outline briefly how to do a flame test:, what are the two main problems with a flame test? Instead, they usually have no visible flame color or may show a pale blue flame due to the presence of. Ammonium compounds do not produce a distinct color. You should also read this: Measurements For Ohio Maneuverability Test

Flame Tests for Unknowns YouTube - Ammonium ions (nh 4+) can be detected in a. The basic premise is that heat from a flame gives atoms enough energy that their electrons become excited. Study with quizlet and memorize flashcards containing terms like what do flame tests do?, outline briefly how to do a flame test:, what are the two main problems with a flame test? Instrumental. You should also read this: Anova_test R