
Iodoform test of acetone YouTube - This reaction is called haloform reaction, and is commonly known. An aldehyde or ketone which has a methyl. To prepare a pure sample of triiodomethyl (iodoform) from acetone. (1) acetone is when treated with iodine and potassium hydroxide, producing iodoform. The only aldehyde that can detect this reaction is acetone because it is the only methyl. You should also read this: Conditions For A Z Test

Iodoform Test Mechanism YouTube - Iodoform, also known as triiodomethane, is a yellow solid with. A pale yellow precipitate known as triiodomethane or iodoform is produced when iodine is added to the unknown compound that contains aldehydes or ketones in the presence of excess sodium hydroxide, and it has an antiseptic odour. During the iodoform reaction of acetone why does the second iodine addition happen. You should also read this: Water Testing Certificate For The Delray Beach Fl33446
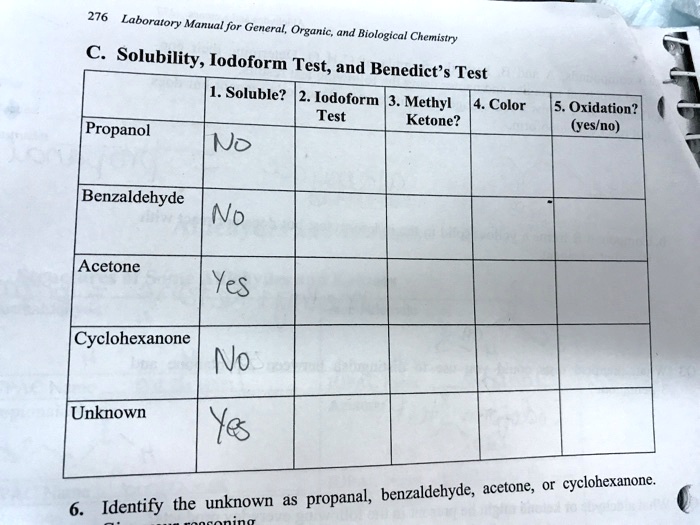
SOLVED 276 Laboratory Manual for General, Organic, and Biological - This is because acetone is a methyl ketone, which meets the structural criteria necessary for the test. Iodoform tests using l2and naoh are more commonly used. Acetic acid derivatives such as ethyl acetate have been considered to be negative to the iodoform test because of the predominant hydrolysis leading to acetic acid. Acetone undergoes the iodoform test by reacting with. You should also read this: Modafinil Drug Test

iodoform test of Acetone test of COCH3 Group YouTube - A pale yellow precipitate known as triiodomethane or iodoform is produced when iodine is added to the unknown compound that contains aldehydes or ketones in the presence of excess sodium hydroxide, and it has an antiseptic odour. Does not give iodoform test because it has two ethyl groups attached to carbonyl groups. One might also ask, does acetone provide iodoform. You should also read this: Maryland Permit Test Questions And Answers
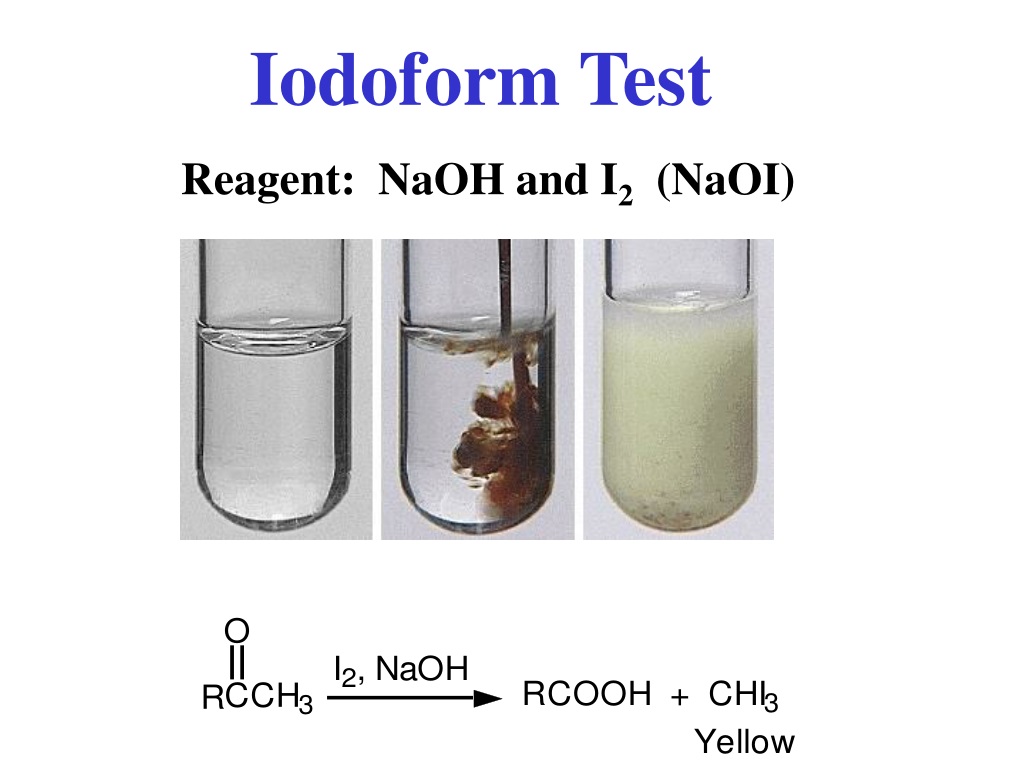
PPT Type of compound PowerPoint Presentation, free download ID9408601 - This experiment aims to prepare iodoform from acetone using iodine and sodium hydroxide solution. During the iodoform reaction of acetone why does the second iodine addition happen on the carbon that already has undergone substitution instead of the other carbon?. Iodoform, also known as triiodomethane, is a yellow solid with. An aldehyde or ketone which has a methyl. (1) acetone. You should also read this: The Giver Ar Test Answers

Iodoform Test Reaction Definition, Mechanism, Examples - Does not give iodoform test because it has two ethyl groups attached to carbonyl groups. When reaction takes place in following steps. This is because acetone is a methyl ketone, which meets the structural criteria necessary for the test. Acetone undergoes the iodoform test by reacting with iodine and aqueous sodium hydroxide (naoh) to form sodium acetate (ch 3 coona). You should also read this: Eclectic Energies Chakra Test

Iodoform Test for acetone class 12 chemistry YouTube - During the iodoform reaction of acetone why does the second iodine addition happen on the carbon that already has undergone substitution instead of the other carbon?. One might also ask, does acetone provide iodoform testing? This experiment aims to prepare iodoform from acetone using iodine and sodium hydroxide solution. An aldehyde or ketone which has a methyl. Acetic acid derivatives. You should also read this: One Touch Verio Test Strips Compatibility

6.4 Detection of acetone in urine (iodoform test) YouTube - A pale yellow precipitate known as triiodomethane or iodoform is produced when iodine is added to the unknown compound that contains aldehydes or ketones in the presence of excess sodium hydroxide, and it has an antiseptic odour. Iodoform tests using l2and naoh are more commonly used. One might also ask, does acetone provide iodoform testing? An aldehyde or ketone which. You should also read this: Cap Achievement 6 Drill Test

A compound that gives a positive iodoform test is - Yes, acetone does give a positive iodoform test. In this experiment, you will be asked to identify an unknown liquid, which will be either an alcohol, aldehyde, or ketone. Acetic acid derivatives such as ethyl acetate have been considered to be negative to the iodoform test because of the predominant hydrolysis leading to acetic acid. During the iodoform reaction of. You should also read this: Arkray Glucocard Expression Test Strips

Iodoform test (acetone identifying reaction) YouTube - Iodoform is obtained by the action of iodine on ethanol (ethyl alcohol), or on propanone (acetone) in the presence of an alkali. During the iodoform reaction of acetone why does the second iodine addition happen on the carbon that already has undergone substitution instead of the other carbon?. (1) acetone is when treated with iodine and potassium hydroxide, producing iodoform.. You should also read this: Placement Test Sinclair